Stereo-Differentiating Asymmetric Rh(I)-Catalyzed Pauson-Khand Reaction: A DFT-Informed Approach to Thapsigargin Stereoisomers
- PMID: 39702925
- PMCID: PMC11726561
- DOI: 10.1021/jacs.4c11661
Stereo-Differentiating Asymmetric Rh(I)-Catalyzed Pauson-Khand Reaction: A DFT-Informed Approach to Thapsigargin Stereoisomers
Abstract
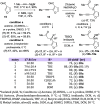
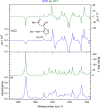
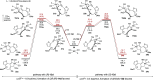
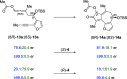
We report a stereo-differentiating dynamic kinetic asymmetric Rh(I)-catalyzed Pauson-Khand reaction, which provides access to an array of thapsigargin stereoisomers. Using catalyst-control, a consistent stereochemical outcome is achieved at C2─for both matched and mismatched cases─regardless of the allene-yne C8 stereochemistry. The stereochemical configuration for all stereoisomers was assigned by comparing experimental vibrational circular dichroism (VCD) and 13C NMR to DFT-computed spectra. DFT calculations of the transition-state structures corroborate experimentally observed stereoselectivity and identify key stabilizing and destabilizing interactions between the chiral ligand and allene-yne PKR substrates. The robust nature of our catalyst-ligand system places the total synthesis of thapsigargin and its stereoisomeric analogues within reach.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Computationally Guided Catalyst Design in the Type I Dynamic Kinetic Asymmetric Pauson-Khand Reaction of Allenyl Acetates.J Am Chem Soc. 2017 Oct 25;139(42):15022-15032. doi: 10.1021/jacs.7b07121. Epub 2017 Oct 12. J Am Chem Soc. 2017. PMID: 29022341
-
Rh(I)-Catalyzed Allenic Pauson-Khand Reaction to Access the Thapsigargin Core: Influence of Furan and Allenyl Chloroacetate Groups on Enantioselectivity.Org Lett. 2022 Feb 4;24(4):995-999. doi: 10.1021/acs.orglett.1c03951. Epub 2022 Jan 26. Org Lett. 2022. PMID: 35081313
-
Systematic Parameter Determination Aimed at a Catalyst-Controlled Asymmetric Rh(I)-Catalyzed Pauson-Khand Reaction.ACS Catal. 2024 Nov 5;14(22):17065-17076. doi: 10.1021/acscatal.4c04490. eCollection 2024 Nov 15. ACS Catal. 2024. PMID: 39569153 Free PMC article.
-
Catalyst Optimisation for Asymmetric Synthesis by Ligand Chirality Element Addition: A Perspective on Stereochemical Cooperativity.Chemistry. 2017 Aug 25;23(48):11460-11478. doi: 10.1002/chem.201700926. Epub 2017 Jul 24. Chemistry. 2017. PMID: 28419592 Review.
-
Pauson-Khand reaction of fluorinated compounds.Beilstein J Org Chem. 2020 Jul 14;16:1662-1682. doi: 10.3762/bjoc.16.138. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32733610 Free PMC article. Review.
Cited by
-
Dynamic Kinetic Asymmetric Hydroacylation: Racemization by Soft Enolization.J Am Chem Soc. 2025 May 14;147(19):16270-16281. doi: 10.1021/jacs.5c01753. Epub 2025 Apr 29. J Am Chem Soc. 2025. PMID: 40298317 Free PMC article.
References
-
- Chen S.; Jiang C.; Zheng N.; Yang Z.; Shi L. Evolution of Pauson-Khand reaction: Strategic applications in total syntheses of architecturally complex natural products (2016–2020). Catalysts 2020, 10 (10), 1199.10.3390/catal10101199. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

