Critical roles of extracellular vesicles in periodontal disease and regeneration
- PMID: 39703170
- PMCID: PMC11954511
- DOI: 10.1093/stcltm/szae092
Critical roles of extracellular vesicles in periodontal disease and regeneration
Abstract
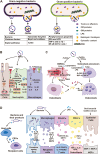
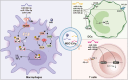
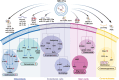
Extracellular vesicles (EVs) are evolutionarily conserved communication mediators that play key roles in the development of periodontal disease as well as in regeneration processes. This concise review first outlines the pathogenic mechanisms through which EVs derived from bacteria lead to the progression of periodontitis, with a focus on the enrichment of virulence factors, the amplification of immune responses, and the induction of bone destruction as key aspects influenced by bacterial EVs. This review aims to elucidate the positive effects of EVs derived from mesenchymal stem cells (MSC-EVs) on periodontal tissue regeneration. In particular, the anti-inflammatory properties of MSC-EVs and their impact on the intricate interplay between MSCs and various immune cells, including macrophages, dendritic cells, and T cells, are described. Moreover, recent advancements regarding the repair-promoting functions of MSC-EVs are detailed, highlighting the mechanisms underlying their ability to promote osteogenesis, cementogenesis, angiogenesis, and the homing of stem cells, thus contributing significantly to periodontal tissue regeneration. Furthermore, this review provides insights into the therapeutic efficacy of MSC-EVs in treating periodontitis within a clinical context. By summarizing the current knowledge, this review aims to provide a comprehensive understanding of how MSC-EVs can be harnessed for the treatment of periodontal diseases. Finally, a discussion is presented on the challenges that lie ahead and the potential practical implications for translating EV-based therapies into clinical practices for the treatment of periodontitis.
Keywords: MSC- mesenchymal stem cells-extracellular vesicles; extracellular vesicles; mesenchymal stem cells; periodontal disease; periodontal regeneration.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
The authors declared no potential conflicts of interests.
Figures


References
-
- Trindade D, Carvalho R, Machado V, et al. Prevalence of periodontitis in dentate people between 2011 and 2020: a systematic review and meta‐analysis of epidemiological studies. J Clin Periodontol. 2023;50:604-626. https://doi.org/ 10.1111/jcpe.13769 - DOI - PubMed
-
- Slots J. Primer on etiology and treatment of progressive/severe periodontitis: a systemic health perspective. Periodontology 2000. 2020;83:272-276. https://doi.org/ 10.1111/prd.12325 - DOI - PubMed
-
- Genco RJ, Sanz M.. Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontology 2000. 2020;83:7-13. https://doi.org/ 10.1111/prd.12344 - DOI - PubMed
-
- Tonetti MS, Jepsen S, Jin L, Otomo‐Corgel J.. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: a call for global action. J Clin Periodontol. 2017;44:456-462. https://doi.org/ 10.1111/jcpe.12732 - DOI - PubMed
-
- Hajishengallis G, Chavakis T.. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol. 2021;21:426-440. https://doi.org/ 10.1038/s41577-020-00488-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

