Characterisation of GPR17-expressing oligodendrocyte precursors in human ischaemic lesions and correlation with reactive glial responses
- PMID: 39703181
- PMCID: PMC11717493
- DOI: 10.1002/path.6381
Characterisation of GPR17-expressing oligodendrocyte precursors in human ischaemic lesions and correlation with reactive glial responses
Abstract
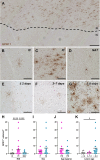
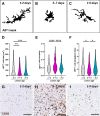
White matter damage and subsequent demyelination significantly contribute to long-term functional impairment after ischaemic stroke. Identifying novel pharmacological targets to restore myelin integrity by promoting the maturation of oligodendrocyte precursor cells (OPCs) into new myelinating oligodendrocytes may open new perspectives for ischaemic stroke treatment. In this respect, previous studies highlighted the role of the G protein-coupled membrane receptor 17 (GPR17) as a key regulator of OPC differentiation in experimental models of brain injury, including ischaemic stroke. To determine the translational value of GPR17 as a possible target in the context of human disease, we exploited immunohistochemistry to characterise the distribution of GPR17-expressing cells in brain tissue samples from ischaemic stroke cases and correlated it with the reactive state of neighbouring glial cells. The results showed that GPR17 specifically decorates a subpopulation of differentiation-committed OPCs, labelled by the peculiar marker breast carcinoma-amplified sequence 1 (BCAS1), that accumulates in the peri-infarct region in the later stages after the ischaemic event. Interestingly, the response of GPR17-expressing cells appears to be paralleled by the switch of reactive microglia/macrophages from a phagocytic to a dystrophic phenotype and by astrocytic scar formation. A negative correlation was found between GPR17-expressing OPCs and reactive microglia/macrophages and astrocytes surrounding chronic ischaemic lesions in female subjects, while the same relationship was less pronounced in males. These results were reinforced by bioinformatic analysis of a publicly available transcriptomic dataset, which implicated a possible role of inflammation and defective neuron-to-OPC communication in remyelination failure after ischaemic damage. Hence, these data strengthen the relevance of GPR17-based remyelinating therapies for the treatment of ischaemic stroke. © 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Keywords: GPR17 receptor; astrocytes; glial cell interactions; ischaemic stroke; microglia; neuroinflammation; oligodendrocyte precursor cells; oligodendrocytes; post‐mortem brain tissue; remyelination.
© 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Figures




References
-
- Marin MA, Carmichael ST. Mechanisms of demyelination and remyelination in the young and aged brain following white matter stroke. Neurobiol Dis 2019; 126: 5–12. - PubMed
-
- Cheng YJ, Wang F, Feng J, et al. Prolonged myelin deficits contribute to neuron loss and functional impairments after ischaemic stroke. Brain 2024; 147: 1294–1311. - PubMed
-
- Boda E, Viganò F, Rosa P, et al. The GPR17 receptor in NG2 expressing cells: focus on in vivo cell maturation and participation in acute trauma and chronic damage. Glia 2011; 59: 1958–1973. - PubMed

