Clinical Profile and Outcomes of Pediatric Snakebite Envenomation: A Three-Year Retrospective Study From a Rural Tertiary Care Center in South India
- PMID: 39703275
- PMCID: PMC11656267
- DOI: 10.7759/cureus.73976
Clinical Profile and Outcomes of Pediatric Snakebite Envenomation: A Three-Year Retrospective Study From a Rural Tertiary Care Center in South India
Abstract
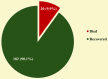
Background Snakebite envenomation remains a significant public health challenge in tropical countries, particularly affecting the pediatric population. Children are especially vulnerable because of their smaller body mass, outdoor activities, and delayed presentation to healthcare facilities. This study aimed to analyze the clinical profile, demographic patterns, and envenomation characteristics of snakebites in children aged 1-16 years presenting to a tertiary care center. Additionally, the study sought to evaluate the spectrum of complications and clinical outcomes in pediatric snakebite cases while assessing the mortality rate and associated risk factors in pediatric snakebite envenomation. Methodology A retrospective medical record review was conducted analyzing all snakebite cases in children aged below 16 years admitted to the Department of Pediatrics, Dhanalakshmi Srinivasan Medical College and Hospital, Perambalur, Tamil Nadu (southern state in India), between January 2021 and December 2023. Data extracted included demographics, bite characteristics, clinical manifestations, time to healthcare presentation, and treatment details. Management protocols followed World Health Organization (WHO) guidelines for snakebite envenomation, including the administration of polyvalent anti-snake venom (ASV) when indicated. Results Among 202 pediatric snakebite cases, children aged 9-12 years constituted the majority (n = 110, 54.5%), with significant male predominance (n = 148, 73.3%). Unidentified snakes were responsible for the highest proportion of bites (n = 72, 35.6%), followed by vipers (n = 65, 32.2%) and cobras (n = 53, 26.2%). Lower limb bites were most frequent (n = 108, 53.5%), and seasonal analysis revealed peak incidence during January-April (n = 106, 52.5%). Common clinical manifestations included hematuria (n = 112, 55.4%), oliguria (n = 102, 50.5%), and renal failure (n = 80, 39.6%). Most patients (n = 120, 59.4%) received antivenom within six hours, with 46.6% (n = 94) requiring 5-10 vials. The overall mortality rate was 9.9% (n = 20). Statistical analysis revealed significant associations between mortality and snake species identification (p = 0.0014), with the highest mortality in unidentified snakebites (n = 15, 20.8%). Anatomical bite site (p = 0.042), renal failure (p = 0.001), respiratory paralysis (p = 0.001), and ptosis (p = 0.001) were also significantly associated with mortality. Time to antivenom administration significantly impacted survival (p = 0.001), with mortality rates of 0.8% (n = 1) for treatment within six hours, increasing to 38.5% (n = 10) for delays beyond 12 hours. Demographics and local manifestations showed no significant correlation with mortality. Conclusion Our study reveals distinct patterns in pediatric snakebites, predominantly affecting male children in pre-adolescent age. The high proportion of unidentified snakes remains challenging. With renal and hematological manifestations being frequent, mortality is significantly influenced by delayed treatment, respiratory paralysis, and renal failure. Early antivenom administration, proper snake identification, and prompt medical intervention remain crucial factors in improving outcomes and reducing mortality.
Keywords: anti-snake venom; neuroparalytic; outcome assessment; snakebite; snakebite envenoming; vasculotoxic.
Copyright © 2024, Muniyapillai et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. The Institutional Ethics Committee of Dhanalakshmi Srinivasan Medical College and Hospital issued approval IECHS/IRCHS/No. 391A. The study protocol received approval from the Institutional Ethics Committee of Dhanalakshmi Srinivasan Medical College (approval number: IECHS/IRCHS/No. 391A) with a waiver of individual informed consent because of its retrospective nature and the use of existing medical records. All patient data were de-identified during data extraction and analysis to maintain confidentiality, with each case assigned a unique study identifier. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
Similar articles
-
A retrospective study of clinico-epidemiological profile of snakebite related deaths at a Tertiary care hospital in Midnapore, West Bengal, India.Toxicol Rep. 2017 Nov 24;5:1-5. doi: 10.1016/j.toxrep.2017.11.008. eCollection 2018. Toxicol Rep. 2017. PMID: 29234603 Free PMC article.
-
A season of snakebite envenomation: presentation patterns, timing of care, anti-venom use, and case fatality rates from a hospital of southcentral Nepal.J Venom Res. 2016 Jan 23;7:1-9. eCollection 2016. J Venom Res. 2016. PMID: 26998219 Free PMC article.
-
Clinical and epidemiologic profile and predictors of outcome of poisonous snake bites - an analysis of 1,500 cases from a tertiary care center in Malabar, North Kerala, India.Int J Gen Med. 2018 Jun 5;11:209-216. doi: 10.2147/IJGM.S136153. eCollection 2018. Int J Gen Med. 2018. PMID: 29892202 Free PMC article.
-
The concept of Big Four: Road map from snakebite epidemiology to antivenom efficacy.Int J Biol Macromol. 2023 Jul 1;242(Pt 1):124771. doi: 10.1016/j.ijbiomac.2023.124771. Epub 2023 May 9. Int J Biol Macromol. 2023. PMID: 37169043 Review.
-
Prospective review of cytotoxic snakebite envenomation in a paediatric population.Toxicon. 2021 Jan 30;190:73-78. doi: 10.1016/j.toxicon.2020.12.009. Epub 2020 Dec 17. Toxicon. 2021. PMID: 33340504 Review.
Cited by
-
Vipera Snakebite in Children: A Focus on Europe.Children (Basel). 2025 Mar 20;12(3):393. doi: 10.3390/children12030393. Children (Basel). 2025. PMID: 40150675 Free PMC article. Review.
References
-
- WHO's snakebite envenoming strategy for prevention and control. Minghui R, Malecela MN, Cooke E, Abela-Ridder B. Lancet Glob Health. 2019;7:0. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous