autoMEA: machine learning-based burst detection for multi-electrode array datasets
- PMID: 39703343
- PMCID: PMC11655478
- DOI: 10.3389/fnins.2024.1446578
autoMEA: machine learning-based burst detection for multi-electrode array datasets
Abstract
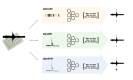
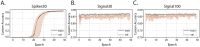
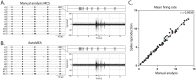
Neuronal activity in the highly organized networks of the central nervous system is the vital basis for various functional processes, such as perception, motor control, and cognition. Understanding interneuronal connectivity and how activity is regulated in the neuronal circuits is crucial for interpreting how the brain works. Multi-electrode arrays (MEAs) are particularly useful for studying the dynamics of neuronal network activity and their development as they allow for real-time, high-throughput measurements of neural activity. At present, the key challenge in the utilization of MEA data is the sheer complexity of the measured datasets. Available software offers semi-automated analysis for a fixed set of parameters that allow for the definition of spikes, bursts and network bursts. However, this analysis remains time-consuming, user-biased, and limited by pre-defined parameters. Here, we present autoMEA, software for machine learning-based automated burst detection in MEA datasets. We exemplify autoMEA efficacy on neuronal network activity of primary hippocampal neurons from wild-type mice monitored using 24-well multi-well MEA plates. To validate and benchmark the software, we showcase its application using wild-type neuronal networks and two different neuronal networks modeling neurodevelopmental disorders to assess network phenotype detection. Detection of network characteristics typically reported in literature, such as synchronicity and rhythmicity, could be accurately detected compared to manual analysis using the autoMEA software. Additionally, autoMEA could detect reverberations, a more complex burst dynamic present in hippocampal cultures. Furthermore, autoMEA burst detection was sufficiently sensitive to detect changes in the synchronicity and rhythmicity of networks modeling neurodevelopmental disorders as well as detecting changes in their network burst dynamics. Thus, we show that autoMEA reliably analyses neural networks measured with the multi-well MEA setup with the precision and accuracy compared to that of a human expert.
Keywords: automated analysis; burst detection; machine learning; multi-electrode array (MEA); neuronal network activity; reverberations.
Copyright © 2024 Hernandes, Heuvelmans, Gualtieri, Meijer, Woerden and Greplova.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Cerina M., Piastra M. C., Frega M. (2023). The potential of in vitro neuronal networks cultured on micro electrode arrays for biomedical research. Progr. Biomed. Eng. 5:032002. 10.1088/2516-1091/acce12 - DOI
LinkOut - more resources
Full Text Sources

