Fecal microbiota transplantation from postmenopausal osteoporosis human donors accelerated bone mass loss in mice
- PMID: 39703374
- PMCID: PMC11655470
- DOI: 10.3389/fcimb.2024.1488017
Fecal microbiota transplantation from postmenopausal osteoporosis human donors accelerated bone mass loss in mice
Abstract
Objectives: To investigate the effect of gut microbiota from postmenopausal osteoporosis patients on bone mass in mice.
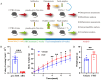
Methods: Fecal samples were collected from postmenopausal women with normal bone mass (Con, n=5) and postmenopausal women with osteoporosis (Op, n=5). Microbial composition was identified by shallow shotgun sequencing. Then fecal samples were transplanted into pseudo-sterile mice previously treated with antibiotics for 4 weeks. These mice were categorized into two groups: the Vehicle group (n=7) received fecal samples from individuals with normal bone mass, and the FMT group (n=7) received fecal samples from individuals with osteoporosis. After 8 weeks, bone mass, intestinal microbial composition, intestinal permeability and inflammation were assessed, followed by a correlation analysis.
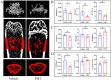
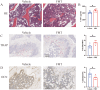
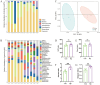
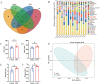
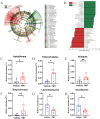
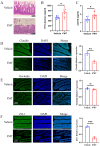
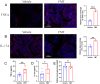
Results: The bone mass was significantly reduced in the FMT group. Microbiota sequencing showed that Shannon index (p < 0.05) and Simpson index (p < 0.05) were significantly increased in Op groups, and β diversity showed significant differences. the recipient mice were similar. linear discriminant analysis effect size (LEfSe) analysis of mice showed that Halobiforma, Enterorhabdus, Alistipes, and Butyricimonas were significantly enriched in the FMT group. Lachnospiraceae and Oscillibacter were significantly enriched in the Vehicle group. H&E staining of intestinal tissues showed obvious intestinal mucosal injury in mice. Intestinal immunohistochemistry showed that the expression of Claudin and ZO-1 in the intestinal tissue of the FMT group mice was decreased. The FITC-Dextran (FD-4) absorption rate and serum soluble CD14 (sCD14) content were increased in FMT mice. Correlation analysis showed that these dominant genera were significantly associated with bone metabolism and intestinal permeability, and were associated with the enrichment of specific enzymes. Serum and bone tissue inflammatory cytokines detection showed that the expression of TNF-α and IL-17A in the FMT group were significantly increased.
Conclusion: Overall, our findings suggested gut microbiota from postmenopausal osteoporosis patients accelerate bone mass loss in mice. Aberrant gut microbiota might play a causal role in the process of bone mass loss mediated by inflammation after the destruction of the intestinal barrier.
Keywords: fecal microbiota transplantation; gut microbiota; inflammation; intestinal permeability; osteoporosis.
Copyright © 2024 Chen, Wang, Hao and Fu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Adeel S., Singh K., Vydareny K. H., Kumari M., Shah E., Weitzmann M. N., et al. (2013). Bone loss in surgically ovariectomized premenopausal women is associated with T lymphocyte activation and thymic hypertrophy. J. Investig. Med. 61, 1178–1183. doi: 10.2310/JIM.0000000000000016 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

