Spontaneous adverse drug reactions reported in a thirteen-year pharmacovigilance program in a tertiary university hospital
- PMID: 39703397
- PMCID: PMC11655218
- DOI: 10.3389/fphar.2024.1427772
Spontaneous adverse drug reactions reported in a thirteen-year pharmacovigilance program in a tertiary university hospital
Abstract
Objectives: We aimed to assess the characteristics of adverse drug reactions (ADRs) collected in a university hospital.
Methods: A retrospective analysis of ADRs spontaneously reported in the Hospital Pharmacovigilance Program database (RutiRAM) over a 13-year period was conducted. The analysis included a description of ADRs [System Organ Class (SOC)] and their seriousness, the drugs involved [level 1 of the Anatomical Therapeutic Chemical (ATC) Classification System], drug-drug interactions, medication errors, drugs 'under additional monitoring', positive rechallenge, and the 'pharmacovigilance interest' of ADRs. An ADR was considered of 'pharmacovigilance interest' when it was serious, and/or produced sequelae, and/or affected the paediatric population, and/or when the suspected drug was 'under additional monitoring'. Additionally, an exploratory analysis for bivariate associations through an automated method was performed.
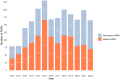
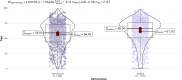
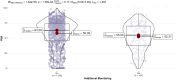
Results: A total of 2,148 spontaneous ADRs were registered in the RutiRAM database, with 92.5% recorded by medical doctors. The mean age of cases was 59.2 years (SD 20.9), range 1 day-99 years; 5.7% were paediatric, 46.2% adults, and 48.1% elderly. The drugs most often involved were anti-infectives (ATC group J), mainly amoxicillin-clavulanic acid. 'Blood system disorders' were the most frequent SOC ADRs, and skin rashes were the most frequent ADRs. The 63.2% of ADRs were considered of 'pharmacovigilance interest'. Almost half of ADRs were hospital-acquired, and these were related to medication error; serious ADRs were related to drug-drug interactions and elderly patients, and involved drugs 'under additional monitoring' were related to younger ones.
Conclusion: This is the first study to overview of ADRs reported in an HPVP over more than a decade. Almost two-thirds of the ADRs collected in the RutiRAM database are of sufficient quality to be classified as 'pharmacovigilance interest', and thus can contribute to signal detection and the issuing of drug alerts by pharmacovigilance systems. Analysing ADRs in hospitals contributes to patient safety by implementing relevant actions to prevent medication errors or ADRs, some of which can be applied to other centres.
Keywords: adverse drug reaction; patient safety; pharmacovigilance; postmarketing drug safety; spontaneous reporting systems.
Copyright © 2024 Montané, Sanz, Martin, Pérez-Mañá, Papaseit, Hladun, De la Rosa and Farré.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- About Medication Errors (2024). National coordinating council for medication error reporting and prevention. Available at: https://www.nccmerp.org/about-medication-errors (Accessed October 23, 2024).
LinkOut - more resources
Full Text Sources
Research Materials

