Cryoablation of primary breast cancer tumors induces a systemic abscopal effect altering TIME (Tumor Immune Microenvironment) in distant tumors
- PMID: 39703517
- PMCID: PMC11657241
- DOI: 10.3389/fimmu.2024.1498942
Cryoablation of primary breast cancer tumors induces a systemic abscopal effect altering TIME (Tumor Immune Microenvironment) in distant tumors
Abstract
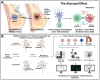
Introduction: Despite recent advances, triple-negative breast cancer (TNBC) patients remain at high risk for recurrence and metastasis, which creates the need for innovative therapeutic approaches to improve patient outcomes. Cryoablation is a promising, less invasive alternative to surgical resection, capable of inducing tumor necrosis via freeze/thaw cycles. Necrotic cell death results in increased inflammatory signals and release of preserved tumor antigens, which have the potential to boost the local and systemic anti-tumor immune response. Thus, compared to surgery, cryoablation enhances the activation of T cells leading to an improved abscopal effect, defined as the occurrence of a systemic response after local treatment. We previously showed with a bilateral-tumor mouse model of TNBC that cryoablation of the primary tumor leads to increased infiltration of distant (abscopal) tumors by tumor infiltrating lymphocytes (TILs) and decreased rates of recurrence and metastasis. However, the early drivers of the cryoablation generated abscopal effect are still unknown and knowledge of the mechanism could provide insight into improving the anti-tumor immune response through pharmacologic immune modulation in addition to cryoablation.
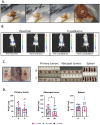
Methods: One million 4T1-12B-luciferase expressing cells were transplanted into the mammary fat pad of BALB/c mice. Two weeks later, left (primary) tumors were either resected or cryoablated. A week after the procedure, right (abscopal) and left tumors, along with spleen, tumor-draining lymph node and blood were collected and processed for flow cytometry and/or RNA-sequencing and immunofluorescence.
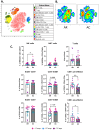
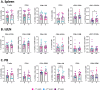
Results: Here we show that cryoablation of mouse mammary carcinomas results in smaller abscopal tumors that harbor increased frequencies of anti-tumor cells [such as natural killer (NK) cells], accompanied by a systemic increase in the frequency of migratory conventional type 1 dendritic cells (cDC1; CD103+ XCR1+), compared to resection. The changes in cell frequencies are mirrored by the immune gene signature of the abscopal tumors, with cryoablation inducing genes involved with NK cell activation and leukocyte-mediated toxicity, including IL11ra1 and Pfr1.
Conclusions: These results better define the early mechanisms through which cryoablation improves tumor elimination, which is mediated by enhanced frequencies of anti-tumoral cells such as NK and cDC1s at the abscopal tumor and in the spleen of mice treated with cryoablation, respectively.
Keywords: RNA-seq analysis; abscopal effect; breast cancer; cryoablation; immune response.
Copyright © 2024 Sardela de Miranda, Martinez-Marin, Babcock, Castro, Boligala, Khan, Furr, Castro-Piedras, Wagner, Robison, Daniele, Singh, Pruitt, Melkus and Layeequr Rahman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Farhangnia P, Akbarpour M. Immunological tolerance. In: Rezaei N, editor. Encyclopedia of Infection and Immunity. Amsterdam, The Netherlands: Elsevier; (2022). p. 206–20.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

