Identifying sex similarities and differences in structure and function of the sinoatrial node in the mouse heart
- PMID: 39703520
- PMCID: PMC11655232
- DOI: 10.3389/fmed.2024.1488478
Identifying sex similarities and differences in structure and function of the sinoatrial node in the mouse heart
Abstract
Background: The sinoatrial node (SN) generates the heart rate (HR). Its spontaneous activity is regulated by a complex interplay between the modulation by the autonomic nervous system (ANS) and intrinsic factors including ion channels in SN cells. However, the systemic and intrinsic regulatory mechanisms are still poorly understood. This study aimed to elucidate the sex-specific differences in heart morphology and SN function, particularly focusing on basal HR, expression and function of hyperpolarization-activated HCN4 and HCN1 channels and mRNA abundance of ion channels and mRNA abundance of ion channels contributing to diastolic depolarization (DD) and spontaneous action potentials (APs).
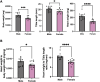
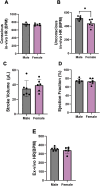
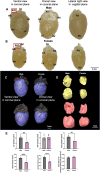
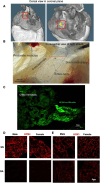
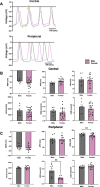
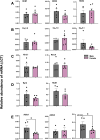
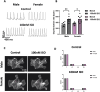
Methods: Body weight, heart weight and tibia length of 2- to 3-month-old male and female mice were measured. Conscious in-vivo HR of male and female mice was recorded via electrocardiography (ECG). Unconscious ex-vivo HR, stroke volume (SV) and ejection fraction (EF) were recorded via echocardiography. Ex-vivo HR was measured via Langendorff apparatus. Volume of atria, ventricles and whole hearts were measured from the ex-vivo hearts by microcomputed tomography (micro-CT). Immunohistochemistry targeting HCN4 and HCN1 was conducted in the SN and RA tissues from both male and female hearts. The funny current (I f) of SN cells in 1 nM and following wash-on of 1 μM isoproterenol (ISO) were recorded via whole cell patch clamp. The APs of SN tissue were recorded via sharp microelectrode and optical mapping of membrane voltage. The relative abundance of mRNAs was measured in male and female mice by qPCR.
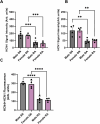
Results: Heart weight to tibia length ratio and heart volume of females were significantly smaller than males. Unconscious in-vivo HR in male mice was higher than that in females. Conscious in-vivo HR, ex-vivo HR, SV, and EF showed no notable difference between male and female mice. Immunohistochemistry revealed HCN4, HCN1, and the sum of HCN4 and HCN1, expression in the SN was notably elevated compared with the RA in both male and females, but there was no sex difference in these channels expression. There were also no significant sex differences in the V 0.5 of I f in SN cells in the presence of 1 nM ISO, however wash-on 1 μM ISO in the same cells induced a significantly increased shift of V 0.5 to more positive voltages in males than in females. The expression of mRNA coding for adrenergic receptor beta-1 (Adrb1) and cholinergic receptors muscarinic 2 (chrm2) in male mice was higher compared with that in female mice. Early diastolic depolarization (EDD) rate in APs from peripheral SN (pSN) from male mice were higher than these in female mice. Mice of both sexes showed equivalent frequency of SN APs and spatial localization of the leading site in control, and similar significant response to ISO 100 nM superfusion.
Conclusion: Males display faster in-vivo HR, but not ex-vivo HR, than females associated with increased expression of Adrb1 in male versus female. This suggests a possible difference in the β-adrenergic modulation in males and females, possibly related to the greater ISO response of I f observed in cells from males. The role of hormonal influences or differential expression of other ion channels may explain these sex-specific variations in HR dynamics. Further investigations are necessary to pinpoint the precise molecular substrates responsible for these differences.
Keywords: diastolic depolarization; electrophysiology; heart rate; ion channels; sex; sinoatrial node.
Copyright © 2024 Yin, Torre, Marrot, Peters, Feather, Nichols, Logantha, Arshad, Martis, Ozturk, Chen, Liu, Qu, Zi, Cartwright, Proenza, Torrente, Mangoni, Dobrzynski and Atkinson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

