Benefit from dose-dense adjuvant chemotherapy for breast cancer: subgroup analyses from the randomised phase 3 PANTHER trial
- PMID: 39703564
- PMCID: PMC11652897
- DOI: 10.1016/j.lanepe.2024.101162
Benefit from dose-dense adjuvant chemotherapy for breast cancer: subgroup analyses from the randomised phase 3 PANTHER trial
Abstract
Background: It is unclear whether some patients with high-risk breast cancer do not warrant adjuvant dose-dense chemotherapy due to small expected absolute benefit.
Methods: The phase 3 PANTHER trial (NCT00798070) compared adjuvant sequential epirubicin/cyclophosphamide (EC) and docetaxel (D) administered in either tailored dose-dense (tDD EC/D) or standard interval schedule (FEC/D) to patients with high-risk resected early breast cancer (n = 2003). We compared outcomes across key subgroups of interest, evaluated the performance of the online prognostication and treatment benefit estimation tool PREDICT and conducted a subpopulation treatment effect pattern plot (STEPP) analysis. Primary endpoint was breast cancer recurrence free survival (BCRFS).
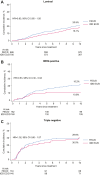
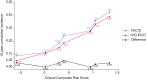
Findings: Median follow-up was 10.3 years. Treatment with tDD EC/D improved 10-year BCRFS across all subgroups including according to menopausal status, with an absolute benefit of 2% or more, as well as in luminal (Hazard Ratio [HR] = 0.83, 95% Confidence Interval [CI] 0.65-1.05) and Human Epidermal Growth Factor Receptor 2 (HER2) positive (HR = 0.53, 95% CI 0.30-0.93), but not triple negative breast cancer patients (HR = 1.02, 95% CI 0.66-1.57). PREDICT underestimated overall survival in the entire population and across all subgroups. In STEPP analysis, absolute benefit from tDD EC/D in BCRFS was stable across risk-defined subpopulations, from 3.8% in the lowest risk patients to 3.6% in the highest risk ones. There was no differential treatment effect over time.
Interpretation: We could not reliably identify any subgroup not benefiting from dose-dense treatment, which should be considered for patients with primary resected high-risk breast cancer.
Funding: Cancerfonden, Bröstcancerförbundet, Radiumhemmets Forskningsfonder, Amgen, Roche, sanofi-aventis.
Keywords: Adjuvant chemotherapy; Amenorrhea; Breast cancer; Dose-dense; PREDICT; STEPP.
© 2024 The Author(s).
Conflict of interest statement
Alexios Matikas: speaker/consultancy (no personal fees) to Veracyte, Roche, Seagen; research funding paid to institution by Merck, AstraZeneca, Novartis, Veracyte. Sibylle Loibl: employment as Chief Executive Officer (CEO) at German Breast Group (GBG) Forschungs GmbH; institutional fees for advisory board membership for AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb (BMS), Celgene, DSI, EirGenix, Gilead, GSK, Lilly, Merck, Novartis, Olema, Pfizer, Pierre Fabre, Relay Therapeutics, Roche, Sanofi and Seagen; institutional fees as an invited speaker for AstraZeneca, DSI, Gilead, Medscape, Novartis, Pfizer, Roche, Seagen and Stemline-Menarini; institutional research grants from AbbVie, AstraZeneca, BMS/Celgene, Daiichi Sankyo, Immunomedics/Gilead, Molecular Health, Stemline-Menarini, Novartis, Pfizer and Roche; institutional funding from Greenwich Life Sciences; institutional licensing fees from VMscope GmbH; a role as a steering committee member (non-financial interest) for AstraZeneca, Daiichi Sankyo, Immunomedics/Gilead, Novartis, Pfizer, Roche and Seagen; a role as a Principal Investigator (PI) for Pfizer, AstraZeneca (non-financial interest). Günther Steger: personal fees and non-financial support from Roche, personal fees and non-financial support from AstraZeneca, personal fees and non-financial support from Novartis, personal fees from Lilly, non-financial support from TEVA, personal fees and non-financial support from Pfizer. Michael Untch: personal fees for lectures and/or consultancy from Agendia, AstraZeneca, Daiichi Sankyo, Eisai Gilead, Lilly Deutschland, MSD, Myriad Genetics, Novartis, Pierre Fabre, Pfizer, Roche, Sanofi Aventis, Seagen, Stemline Richard Greil: institutional grants for research and studies from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, Abbvie, Gilead, and Daiichi Sankyo, and honoraria for lectures or consultancy from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, Abbvie, Gilead, Daiichi Sankyo, and Sanofi. Michael Gnant: personal fees for advisory board membership for Eli Lilly, MSD, Novartis and Menarini-Stemline; personal fees as an invited speaker for AstraZeneca, Daiichi Sankyo, Eli Lilly, EPQ Health, Novartis and Pierre Fabre; personal fees for an expert testimony for Veracyte; membership of the Board of Directors at Austrian Breast and Colorectal Cancer Study Group (ABCSG) GmbH and ABCSG Research Services GmbH; a role as a steering committee member for AstraZeneca (non-financial interest) and Eli Lilly (non-financial interest); a role as trial Chair for Pfizer (non-financial interest); and spouse employment at Sandoz. Theodoros Foukakis: institutional fees for consultancy to AstraZeneca, Daiichi Sankyo, Gilead and Roche; personal fees for consultancy to Affibody, Pfizer, Novartis, Veracyte, Exact Sciences; honoraria from UpToDate; research funding to institution from Pfizer, AstraZeneca, Novartis and Veracyte. Jonas Bergh: research funding to institution from Amgen, AstraZeneca, Bayer, Merck, Pfizer, Roche and Sanofi; honoraria from UpToDate paid to Asklepios Cancer Research AB; head of advisory board at Stratipath AB; Coronis and Asklepios Cancer Research AB hold shares of Stratipath AB; honoraria for lectures/educational conferences for postgraduates courses from AstraZeneca paid to Coronis and Asklepios Cancer Research AB. All the other authors had no potential conflicts of interest to disclos
Figures

References
-
- Matikas A., Foukakis T., Bergh J. Dose intense, dose dense and tailored dose adjuvant chemotherapy for early breast cancer: an evolution of concepts. Acta Oncol. 2017;56(9):1143–1151. - PubMed
-
- National Comprehensive Cancer Network Breast cancer (Version 4.2024) https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
-
- Loibl S., Andre F., Bachelot T., et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up(dagger) Ann Oncol. 2024;35(2):159–182. - PubMed
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

