Loss of Sult1a1 reduces body weight and increases browning of white adipose tissue
- PMID: 39703864
- PMCID: PMC11656314
- DOI: 10.3389/fendo.2024.1448107
Loss of Sult1a1 reduces body weight and increases browning of white adipose tissue
Abstract
Background and objective: Overweight and obesity affects millions of individuals worldwide and consequently represents a major public health concern. Individuals living with overweight and obesity have difficulty maintaining a low body weight due to known physiological mechanisms which prevent further weight loss and drive weight regain. In contrast, mechanisms which promote low body weight maintenance receive less attention and are largely unknown. To uncover these intrinsic mechanisms, we investigated a human cohort of constitutionally thin (CT) individuals which maintain a low body weight and are resistant to weight gain despite exposure to an obesogenic environment.
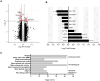
Methods: To identify novel genes that contribute to low body weight maintenance, we performed transcriptomics on adipose tissue biopsies collected from CT and normal body weight (NBW) individuals and identified sulfotransferase 1A1 (SULT1A1) as a target for further investigation in mice. Sult1a1 knockout (KO) mice were fed a standard diet to assess the impact of Sult1a1 deletion on metabolic traits. To determine if high-fat feeding recapitulated the CT weight gain resistance phenotype, Sult1a1 KO mice were fed a high-fat diet for 13-weeks. A subset of wild-type and Sult1a1 KO mice from the standard diet were further analyzed for characterization of adipose tissue respiratory capacity.
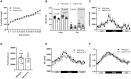
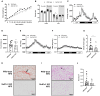
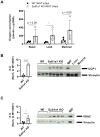
Results: In comparison to NBW controls, adipose tissue from CT individuals expresses less SULT1A1. Sult1a1 KO mice weigh 10% less at the end of the study period and on a high-fat diet, Sult1a1 KO mice tended to gain less weight and had reduced fat mass at 14-weeks of age. These changes were associated with reduced fasting insulin and lessened adipose tissue inflammation and fibrosis. Subcutaneous adipose tissue from Sult1a1 KO mice on a standard chow diet had elevated leak respiration, uncoupling protein 1 (UCP1) expression and increased expression of a mitochondrial marker, VDAC, associating Sult1a1 deletion to adipose tissue browning.
Conclusions: Our results associate Sult1a1 deletion with a tendency for lower body weight through remodeling of white adipose tissue towards a brown phenotype. The presence of UCP1, the expression of an additional mitochondrial protein and increased respiratory capacity suggest browning of the subcutaneous adipose tissue depot of Sult1a1 KO mice.
Keywords: browning; leanness; obesity; sulfotransferase 1A1; white adipose tissue.
Copyright © 2024 Springer, Meugnier, Schnabl, Hof, Champy, Sorg, Petit-Demoulière, Germain, Galusca, Estour, Vidal, Klingenspor and Hager.
Conflict of interest statement
Author MS was employed and author JH is employed by the company Société des Produits Nestlé S.A. KSH is the founder of Maven Health. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

