αFAP-specific nanobodies mediate a highly precise retargeting of modified AAV2 capsids thereby enabling specific transduction of tumor tissues
- PMID: 39703904
- PMCID: PMC11655695
- DOI: 10.1016/j.omtm.2024.101378
αFAP-specific nanobodies mediate a highly precise retargeting of modified AAV2 capsids thereby enabling specific transduction of tumor tissues
Abstract
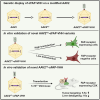
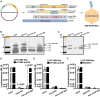
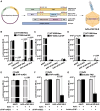
Due to the refractiveness of tumor tissues to adeno-associated virus (AAV) transduction, AAV vectors are poorly explored for cancer therapy delivery. Here, we aimed to engineer AAVs to target tumors by enabling the specific engagement of fibroblast activation protein (FAP). FAP is a cell surface receptor distinctly upregulated in the reactive tumor stroma, but rarely expressed in healthy tissues. Thus, targeting FAP presents an opportunity to selectively transduce tumor tissues. To achieve this, we modified the capsid surface of AAV2 with an αFAP nanobody to retarget the capsid to engage FAP receptor. Following transduction, we observed a 23- to 80-fold increase in the selective transduction of FAP+ tumor cells in vitro, and greater than 5-fold transduction of FAP+ tumor tissues in vivo. Subsequent optimization of the VP1-nanobody expression cassette further enhanced the transduction efficiency of the modified capsids. Due to the limited αFAP nanobodies repertoires, we broadened the versatility of this high-fidelity platform by screening a naive VHH yeast display library, leading to the identification of several novel αFAP nanobody candidates (KD = 0.1 to >100 nM). Hence, our study offers new opportunity for the application of AAV vectors for highly selective delivery of therapeutics to the tumor stroma.
Keywords: AAV; FAP; adeno-associated virus vectors; capsid engineering; fibroblast activation protein; targeted AAV delivery; tumor microenvironment; tumor targeting.
© 2024 The Authors.
Conflict of interest statement
O.O., F.H., P.M., U.M., and F.d.P. are employees of Boehringer Ingelheim Pharma GmbH & Co. KG, Biberach, Germany. P.C., C.K., and T.P. are employees of Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

