Insights and progress on the biosynthesis, metabolism, and physiological functions of gamma-aminobutyric acid (GABA): a review
- PMID: 39703920
- PMCID: PMC11657192
- DOI: 10.7717/peerj.18712
Insights and progress on the biosynthesis, metabolism, and physiological functions of gamma-aminobutyric acid (GABA): a review
Abstract
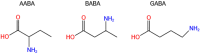
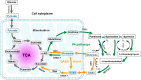
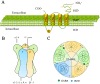
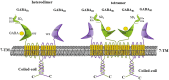
GABA (γ-aminobutyric acid) is a non-protein amino acid that occurs naturally in the human brain, animals, plants and microorganisms. It is primarily produced by the irreversible action of glutamic acid decarboxylase (GAD) on the α-decarboxylation of L-glutamic acid. As a major neurotransmitter in the brain, GABA plays a crucial role in behavior, cognition, and the body's stress response. GABA is mainly synthesized through the GABA shunt and the polyamine degradation pathways. It works through three receptors (GABAA, GABAB, and GABAC), each exhibiting different pharmacological and physiological characteristics. GABA has a variety of physiological roles and applications. In plants, it regulates growth, development and stress responses. In mammals, it influences physiological functions such as nervous system regulation, blood pressure equilibrium, liver and kidneys enhancement, hormone secretion regulation, immunity enhancement, cancer prevention, as well as anti-aging effects. As a biologically active ingredient, GABA possesses unique physiological effects and medicinal value, leading to its widespread application and substantially increased market demand in the food and pharmaceutical industries. GABA is primarily produced through chemical synthesis, plant enrichment and microbial fermentation. In this review, we first make an overview of GABA, focusing on its synthesis, metabolism, GABA receptors and physiological functions. Next, we describe the industrial production methods of GABA. Finally, we discuss the development of ligands for the GABA receptor binding site, the prospects of GABA production and application, as well as its clinical trials in potential drugs or compounds targeting GABA for the treatment of epilepsy. The purpose of this review is to attract researchers from various fields to focus on GABA research, promote multidisciplinary communications and collaborations, break down disciplinary barriers, stimulate innovative research ideas and methods, and advance the development and application of GABA in medicine, agriculture, food and other fields.
Keywords: Biosynthesis; Metabolism; Neurotransmitter; Physiological function; γ-Aminobutyric acid.
© 2024 Zhang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Adachi Y, Toyoshima K, Nishimoto R, Ueno S, Tanaka T, Imaizumi A, Arashida N, Nakamura M, Abe Y, Hakamada T, Kaneko E, Takahashi S, Jinzu H, Shimokado K. Association between plasma alpha-aminobutyric acid and depressive symptoms in older community-dwelling adults in Japan. Geriatrics & Gerontology International. 2019;19(3):254–258. doi: 10.1111/ggi.13585. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

