Evaluation of MAGE-A4 expression in breast cancer and its impact on prognosis
- PMID: 39704015
- PMCID: PMC11875791
- DOI: 10.1111/cas.16433
Evaluation of MAGE-A4 expression in breast cancer and its impact on prognosis
Abstract
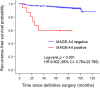
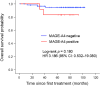
Melanoma-associated antigen (MAGE)-A4, a cancer testis antigen, presents a promising target for chimeric antigen receptor T cell therapy in refractory solid tumors, including breast cancer (BC). However, the lack of highly specific Abs against MAGE-A4 is a major challenge for the development of MAGE-A4-targeted immunotherapies. This study aimed to validate the specificity of a novel MAGE-A4 Ab (E701U) and examine MAGE-A4 expression in clinical BC samples. MAGE-A1, -A2B, -A3, -A4, -A6, -A9, -A10, and -A12 genes were transfected into HEK293 cells. MAGE-A4 expression in each inserted cell block was evaluated using an E701U Ab. Subsequently, we evaluated MAGE-A4 expression in 403 primary BC tissue samples by immunohistochemistry using E701U and analyzed the clinical impact of MAGE-A4 in patients with early BC. The results showed that MAGE-A4 expression was limited to cells transduced with the MAGE-A4 gene. MAGE-A4 expression was observed in 5.7% of the BC samples. Positivity in triple-negative BC was significantly higher than in the other subtypes. The 5-year overall survival rate of patients with MAGE-A4(+) was significantly worse than those with MAGE-A4(-) BC. Moreover, the 5-year recurrence-free survival (RFS) rate of patients with MAGE-A4(+) BC was significantly lower than that of patients with MAGE-A4(-) BC. MAGE-A4 expression was an independent prognostic factor for RFS. In conclusion, the E701U Ab showed reliable specificity for MAGE-A4 expression among MAGE family genes. Patients with MAGE-A4(+) BC have an unfavorable prognosis and represent potential candidates for MAGE-A4-specific immunotherapy.
Keywords: MAGE‐A4; breast cancer; immunotherapy; prognosis; triple‐negative breast cancer.
© 2024 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Y.M. belongs to the Department of Personalized Cancer Immunotherapy at the Mie University Graduate School of Medicine. This is an endowment department supported by a grant from T Cell Nouveau. Y.M. received research funds from ASAHI KASEI Corporation (Japan), Kohjin Bio Co. Ltd. (Japan), and T cell Nouveau Inc. (Japan). The other authors declare no conflicts of interest.
Figures




References
-
- van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643‐1647. - PubMed
-
- Marcar L, Maclaine NJ, Hupp TR, Meek DW. Mage‐a cancer/testis antigens inhibit p53 function by blocking its interaction with chromatin. Cancer Res. 2010;70:10362‐10370. - PubMed
-
- Yang B, O'Herrin SM, Wu J, et al. MAGE‐A, mMage‐b, and MAGE‐C proteins form complexes with KAP1 and suppress p53‐dependent apoptosis in MAGE‐positive cell lines. Cancer Res. 2007;67:9954‐9962. - PubMed
-
- Chomez P, De Backer O, Bertrand M, De Plaen E, Boon T, Lucas S. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 2001;61:5544‐5551. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

