Fasting substrates predict chronic kidney disease progression in CREDENCE trial patients with type 2 diabetes
- PMID: 39704168
- PMCID: PMC11665565
- DOI: 10.1172/jci.insight.180637
Fasting substrates predict chronic kidney disease progression in CREDENCE trial patients with type 2 diabetes
Abstract
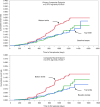
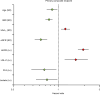
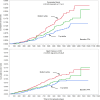
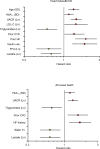
BACKGROUNDSodium-glucose cotransporter 2 inhibitors slow down progression of chronic kidney disease (CKD). We tested whether the circulating substrate mix is related to CKD progression and cardiovascular outcomes in patients with type 2 diabetes (T2D) and albuminuric CKD in the CREDENCE trial.METHODSWe measured fasting substrates in 2,543 plasma samples at baseline and 1 year after randomization to either 100 mg canagliflozin or placebo and used multivariate Cox models to explore their association with CKD progression, heart failure hospitalization/cardiovascular death (hHF/CVD), and mortality.RESULTSHigher baseline lactate and free fatty acids (FFAs) were independently associated with a lower risk of CKD progression (HR = 0.73 [95% CI: 0.54-0.98] and HR = 0.67 [95% CI: 0.48-0.95], respectively) and hHF/CVD HR = 0.70 [95% CI: 0.50-0.99] and HR = 0.63 [95% CI: 0.42-0.94]). Canagliflozin led to a rise in plasma FFAs, glycerol, β-hydroxybutyrate, and acetoacetate. Changes in substrate between baseline and year 1 predicted an approximately 30% reduction in relative risk of both CKD progression and hHF/CVD independently of treatment. More patients who did not respond to canagliflozin treatment in terms of CKD progression belonged to the bottom lactate and FFA distribution tertiles.CONCLUSIONIn T2D patients with albuminuric CKD, basic energy substrates selectively influenced major long-term endpoints; canagliflozin treatment amplified their effects by chronically raising their circulating levels.
Keywords: Cardiology; Diabetes; Drug therapy; Endocrinology; Intermediary metabolism.
Conflict of interest statement
Figures

References
-
- Cosentino F, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. 2020;142(23):2205–2215. doi: 10.1161/CIRCULATIONAHA.120.050255. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

