RTN1A mediates diabetes-induced AKI-to-CKD transition
- PMID: 39704174
- PMCID: PMC11665580
- DOI: 10.1172/jci.insight.185826
RTN1A mediates diabetes-induced AKI-to-CKD transition
Abstract
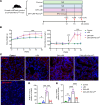
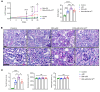
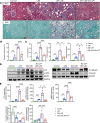
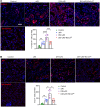
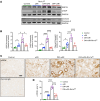
Diabetic patients have increased susceptibility to acute kidney injury (AKI), and AKI could progress to chronic tubulointerstitial injury and fibrosis, referred to as AKI-to-chronic kidney disease (AKI-to-CKD) transition. However, whether diabetes directly promotes AKI-to-CKD transition is not known. We previously showed that reticulon-1A (RTN1A), a gene highly upregulated in injured renal tubular epithelial cells (RTECs), promotes AKI-to-CKD transition in nondiabetic settings. Therefore, we also examined whether reducing RTN1A expression could attenuate diabetes-induced AKI-to-CKD transition. Diabetes was induced by a high-fat diet and streptozotocin injections, and unilateral ischemic reperfusion injury was created as an AKI model in control, diabetic, and RTEC-specific Rtn1a-knockdown diabetic mice. AKI induced greater renal function decline, tubulointerstitial injury, and fibrosis in diabetic mice than in nondiabetic mice. Reduction of RTN1A markedly reduced the CKD development following AKI in diabetic mice, which was associated with reduced ER stress and mitochondrial dysfunction in RTECs. These findings indicate that diabetes markedly accelerates AKI-to-CKD transition and that RTN1A is a crucial mediator of diabetes-induced AKI-to-CKD transition. The development of RTN1A inhibitors could potentially attenuate AKI-to-CKD transition in diabetic patients.
Keywords: Chronic kidney disease; Diabetes; Mitochondria; Nephrology.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

