New insights into the role of ubiquitination in angiogenesis (Review)
- PMID: 39704208
- PMCID: PMC11670862
- DOI: 10.3892/ijmm.2024.5473
New insights into the role of ubiquitination in angiogenesis (Review)
Abstract
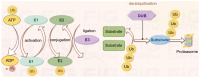
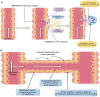
Angiogenesis is a dynamic and complex mechanism for generating new blood vessels from existing ones. Angiogenesis occurs through all life stages and involves several physiological processes. It has an important physiological and pathological role including in cancer, wound healing and inflammation. The emerging role of ubiquitination in regulating angiogenesis highlights the importance of studying this pathway in an angiogenic setting. In angiogenic events, imbalances between pro‑ and anti‑angiogenic factors, induction of hypoxic signaling and stimulation of angiogenic signaling pathways play a central role. This review provides a comprehensive overview of the role of ubiquitination in angiogenesis. This includes angiogenic factors [VEGF, platelet‑derived growth factor, (basic) fibroblast growth factor and angiopoietin], vascular cells (pericytes, endothelial cells, vascular smooth muscle cells) and extracellular matrix and cell adhesion molecules, all of which have important roles in angiogenesis, hypoxic signaling (hypoxia‑inducible factor), which induces angiogenesis, and important vascular signaling pathways (Wnt and Notch). In addition, the molecular biological basis of angiogenesis is discussed and the potential therapeutic value of ubiquitination in angiogenesis‑related diseases is highlighted.
Keywords: Notch signaling molecules; Wnt signaling molecules; angiogenesis; angiogenic factors; cell adhesion molecules; extracellular matrix; hypoxia signaling; ubiquitination; vascular cells.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
