Linear endo-ultrasonographic signs of muscularis propria invasion in early rectal cancer
- PMID: 39704808
- PMCID: PMC11662077
- DOI: 10.1007/s10151-024-03073-4
Linear endo-ultrasonographic signs of muscularis propria invasion in early rectal cancer
Abstract
Background and study aim: Local resection of early rectal cancer is being increasingly used. With invasion of the muscularis propria layer of the rectal wall, the risk of lymph node metastasis becomes too high to consider this the optimal oncological treatment. Therefore, a diagnosis of muscular invasion is important before attempting local resection; however, endoscopic and magnetic resonance imaging (MRI) images have limitations, such as overstaging (26-31%). We investigated the potential of linear endoscopic ultrasound (L-EUS) in the diagnosis of muscularis propria invasion.
Patients and methods: The study consisted of a development phase, in which linear (L)- EUS features, associated with muscular wall invasion were searched and tested, and a validation phase, during which 30 representative videos were assessed by the author F.t.B. and four experienced endosonographists without experience in rectal L-EUS.
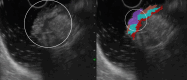
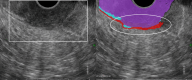
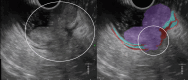
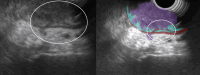
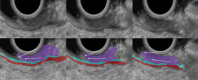
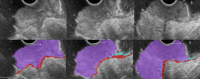
Results: The development cohort consisted of 91 patients (2019-2023). Overall, six EUS features were found to be significantly associated with muscular wall invasion: tornado sign, blob sign, massive connection, layer split, extramural deposit, and, most importantly impaired shiftability between the lesion and muscularis propria layer. During the development phase, these findings demonstrated excellent diagnostic features (sensitivity, 94.4%; specificity, 97.9%; and overstaging, 4%). In the validation phase, the sensitivity, specificity, and overstaging by F.t.B. were 88%, 85%, and 12%, respectively. Among the four inexperienced reviewers, the percentages were 65%-71%, 46%-54%, and 33%-39%, respectively. When considering the 27 videos that were considered easy or moderately difficult to assess, only 55% were correctly interpreted by the inexperienced reviewers.
Conclusions: Linear endoscopic ultrasonography may be a valuable tool for the assessment of ingrowth into the muscularis propria in supposedly early rectal cancer, especially using its dynamic potential to assess fixation to the muscular wall by moving the lesion. However, training will be required to achieve satisfactory results.
Keywords: Endoscopic ultrasound; Oncological staging; Rectal cancer; Tumor infiltration depth.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Given the use of existing data, the study was not subject to the Medical Research Involving Humans Act. All patients gave written consent to the anonymous use of clinical data and EUS media for publication. Informed consent: For this type of study, formal consent is not required.
Figures


References
-
- Toes-Zoutendijk E, van Leerdam ME, Dekker E, van Hees F, Penning C, Nagtegaal I, van der Meulen MP, van Vuuren AJ, Kuipers EJ, Bonfrer JMG, Biermann K, Thomeer MGJ, van Veldhuizen H, Kroep S, van Ballegooijen M, Meijer GA, de Koning HJ, Spaander MCW, Lansdorp-Vogelaar I; Dutch National Colorectal Cancer Screening Working Group (2017) Real-time monitoring of results during first year of dutch colorectal cancer screening program and optimization by altering fecal immunochemical test cut-off levels. Gastroenterology 152:767–775 - PubMed
-
- Ushigome H, Ohue M, Kitamura M, Nakatsuka S, Haraguchi N, Nishimura J, Yasui M, Wada H, Takahashi H, Omori T, Miyata H, Yano M, Takiguchi S (2020) Evaluation of risk factors for lymph node metastasis in T2 lower rectal cancer to perform chemoradiotherapy after local resection. Mol Clin Oncol 12:390–394 - PMC - PubMed
-
- Dutch Guideline on completion surgery after local excision of colorectal cancer, 27-6-2023. https://richtlijnendatabase.nl/richtlijn/colorectaal_carcinoom_crc/prima...
-
- Tanaka S, Saitoh Y, Matsuda T, Igarashi M, Matsumoto T, Iwao Y, Suzuki Y, Nozaki R, Sugai T, Oka S, Itabashi M, Sugihara KI, Tsuruta O, Hirata I, Nishida H, Miwa H, Enomoto N, Shimosegawa T, Koike K (2021) Evidence-based clinical practice guidelines for management of colorectal polyps. J Gastroenterol. 10.1007/s00535-021-01776-1 - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

