MINFLUX fluorescence nanoscopy in biological tissue
- PMID: 39705311
- PMCID: PMC11670107
- DOI: 10.1073/pnas.2422020121
MINFLUX fluorescence nanoscopy in biological tissue
Abstract
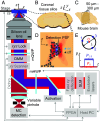
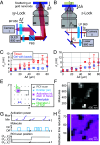
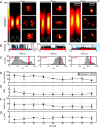
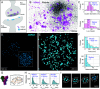
Optical imaging access to nanometer-level protein distributions in intact tissue is a highly sought-after goal, as it would provide visualization in physiologically relevant contexts. Under the unfavorable signal-to-background conditions of increased absorption and scattering of the excitation and fluorescence light in the complex tissue sample, superresolution fluorescence microscopy methods are severely challenged in attaining precise localization of molecules. We reasoned that the typical use of a confocal detection pinhole in MINFLUX nanoscopy, suppressing background and providing optical sectioning, should facilitate the detection and resolution of single fluorophores even amid scattering and optically challenging tissue environments. Here, we investigated the performance of MINFLUX imaging for different synaptic targets and fluorescent labels in tissue sections of the mouse brain. Single fluorophores were localized with a precision of <5 nm at up to 80 µm sample depth. MINFLUX imaging in two color channels allowed to probe PSD95 localization relative to the spine head morphology, while also visualizing presynaptic vesicular glutamate transporter (VGlut) 1 clustering and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) clustering at the postsynapse. Our two-dimensional (2D) and three-dimensional (3D) two-color MINFLUX results in tissue, with <10 nm 3D fluorophore localization, open up broad avenues to investigate protein distributions on the single-synapse level in fixed and living brain slices.
Keywords: 3D nanoscopy; postsynapse; super-resolution microscopy; tissue imaging.
Conflict of interest statement
Competing interests statement:The Max Planck Society holds patents on selected embodiments and procedures of MINFLUX, benefitting S.W.H. S.W.H. owns shares of Abberior Instruments, a company selling MINFLUX microscopes.
Figures



References
-
- Abbe E. C., The relation of aperture and power in the microscope (continued). J. R. Microsc. Soc. 2, 460–473 (1882).
-
- Hell S. W., Microscopy and its focal switch. Nat. Methods 6, 24–32 (2009). - PubMed
-
- Hell S. W., Wichmann J., Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 19, 780–782 (1994). - PubMed
-
- Betzig E., et al. , Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006). - PubMed
MeSH terms
Substances
Grants and funding
- 695568 SYNNOVATE/EC | H2020 | PRIORITY 'Excellent science' | H2020 European Research Council (ERC)
- 885069 SYNAPTOME/EC | H2020 | PRIORITY 'Excellent science' | H2020 European Research Council (ERC)
- 529085/Simons Foundation Autism Research Initiative (SFARI)
- MPSP/Max Planck School of Photonics (MPSP)
LinkOut - more resources
Full Text Sources

