Real-World Safety and Health Care Resource Utilization of Teclistamab Under an Outpatient Model for Step-Up Dosing Administration
- PMID: 39705632
- PMCID: PMC12165516
- DOI: 10.1200/OP-24-00489
Real-World Safety and Health Care Resource Utilization of Teclistamab Under an Outpatient Model for Step-Up Dosing Administration
Abstract
Purpose: Teclistamab is initiated with a step-up dosing (SUD) schedule to mitigate the risk of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Early teclistamab users commonly received SUD in a hospital setting. This study aimed to evaluate safety and health care resource utilization (HRU) in real-world patients with multiple myeloma who initiated teclistamab SUD in an outpatient setting.
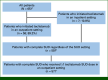
Methods: This was a retrospective study using Mayo Clinic's electronic medical records from October 26, 2022, to October 31, 2023. Patient characteristics were summarized for all patients treated with teclistamab and separately for patients who started SUD outpatient. SUD pattern, safety, HRU, and post-SUD dosing schedule were described in patients with complete SUD.
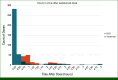
Results: At data cutoff, 65 patients received ≥1 teclistamab dose, including 58 patients who initiated SUD outpatient (median age, 69.2 years; male, 63.8%; White, 89.7%). Among 57 patients who completed SUD in an outpatient setting, all received premedications on the days of teclistamab administrations per label recommendation; 18 (31.6%) developed CRS (13 grade 1, four grade 2, and one grade 4) and two developed ICANS (one each with grade 2 and 4). All CRS and ICANS resolved with supportive care and all patients continued treatment. Eighteen patients were admitted to the hospital for CRS treatment, with a median CRS-related hospital stay of 2 days per admission. Most (60%) doses during SUD required <1 hour clinic time between administration and checkout. Post-SUD, clinic time for treatment doses decreased to <30 minutes for most doses (82%).
Conclusion: Outcomes of this study support outpatient administration as a safe and feasible option for teclistamab SUD to potentially reduce HRU and improve patient experiences.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Munshi NC, Anderson LD Jr, Shah N, et al. : Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med 384:705-716, 2021 - PubMed
-
- Berdeja JG, Madduri D, Usmani SZ, et al. : Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 398:314-324, 2021 - PubMed
-
- Janssen : TECVAYLI [Prescribing Information]. 2024
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

