Neurofilament Light Chain as a Discriminator of Disease Activity Status in MOG Antibody-Associated Disease
- PMID: 39705633
- PMCID: PMC11666271
- DOI: 10.1212/NXI.0000000000200347
Neurofilament Light Chain as a Discriminator of Disease Activity Status in MOG Antibody-Associated Disease
Abstract
Background and objectives: In patients with myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD), acute disease activity is generally identified through medical history, neurologic examination, and imaging. However, these may be insufficient for detecting disease activity in specific conditions. This study aimed to investigate the dynamics of serum neurofilament light chain (sNfL) and serum glial fibrillary acidic protein (sGFAP) after clinical attacks and to assess their utility in discriminating attacks from remission in patients with MOGAD.
Methods: We conducted a multicenter, retrospective, longitudinal study including 239 sera from 62 MOGAD patients assessed from 1995 to 2023 in a discovery and validation setup. Sera were measured for sNfL and sGFAP with a single-molecule array assay and for MOG-IgG with a live cell-based assay. sNfL and sGFAP Z scores and percentiles adjusted for age, body mass index, and sex (sGFAP) were calculated from a healthy control normative database. Mixed-effects regression models were used to characterize biomarkers' dynamics and to investigate associations between serum biomarkers, clinical variables, and disease activity status.
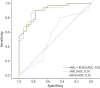
Results: Among the 62 study participants, 29 (46.8%) were female, with a median age at baseline of 40.0 years (interquartile range [IQR] 29.5-49.8) and a median duration of follow-up of 20.0 months (IQR 3.0-62.8). sNfL and sGFAP Z scores were nonlinearly associated with time from attack onset (p < 0.001 and = 0.002, respectively). During attacks, both biomarkers presented higher median values (sNfL Z score 2.9 [IQR 1.4-3.5], 99.8th; sGFAP Z score 0.4 [IQR -0.5 to 1.5], 65.5th) compared with remission (sNfL Z score 0.9 [IQR -0.1 to 1.6], 81.6th, p < 0.001; sGFAP Z score -0.2 [IQR -0.8 to 0.5], 42.1th; p < 0.001) across all clinical phenotypes. sNfL values consistently discriminated disease activity status in the discovery and validation cohorts, showing a 3.5-fold increase in the odds of attacks per Z score unit (odds ratio 3.5, 95% confidence interval 2.3-5.1; p < 0.001). Logistic models incorporating sNfL Z scores demonstrated favorable performance in discriminating disease activity status across both cohorts.
Discussion: sNfL Z scores may serve as a biomarker for monitoring disease activity in MOGAD.
Conflict of interest statement
A.B.A.G.R. Gomes has received a research grant from Roche, paid to the University of São Paulo; S.-H. Kim has lectured, consulted, and received honoraria from Bayer Schering Pharma, Biogen, Genzyme, Merck Serono, and UCB; R. Pretzsch has received travel funds from Teva; A.-C. Lecourt was supported by the Horizon 2020 Eurostar program (grant E!113682); A. Maleska Maceski is an investigator in a phase III clinical trial for the treatment of MOGAD sponsored by Hoffmann-LaRoche; S.L. Apóstolos-Pereira has received research grants and honoraria as a speaker and member of advisory boards by AMGEN/Horizon, Alexion, Biogen, Genzyme, Merck, Novartis, Roche; M. Mehling has received research grants from the Swiss National Science Foundation, Roche, and Merck. His institution (University Hospital Basel) has received fees from his participation on the advisory boards of Merck, Roche, Novartis, and Biogen outside the submitted work; T. Derfuss received speaker fees, research support, travel support, and/or served on Advisory Boards or Steering Committees of Actelion, Alexion, Biogen, Celgene, GeNeuro, MedDay, Merck, Mitsubishi Pharma, Novartis, Roche, and Sanofi-Genzyme; he received research support from Alexion, Biogen, Novartis, Roche, Swiss National Research Foundation, University of Basel, and Swiss MS Society; L. Kappos reported having a patent for Neurostatus UHB-AG with royalties paid Payments made to institution (University Hospital Basel); being CEO of RC2NB (employment by University Hospital Basel), part of the MAGNIMS Steering Committee and a board member of the European Charcot Foundation. J. Kuhle has received speaker fees, research support, travel support, and/or served on advisory boards by Swiss MS Society, Swiss National Research Foundation (320030_189140/1), University of Basel, Progressive MS Alliance, Bayer, Biogen, Bristol Myers Squibb, Celgene, Merck, Novartis, Octave Bioscience, Roche, and Sanofi; H.J. Kim received a grant from the National Research Foundation of Korea and research support from Aprilbio and Eisai; received consultancy/speaker fees from Alexion, Aprilbio, Altos Biologics, Biogen, Celltrion, Daewoong, Eisai, GC Pharma, Handok, Horizon Therapeutics, Kaigene, Kolon Life Science, MDimune, Mitsubishi Tanabe Pharma, Merck Serono, Novartis, Roche, Sanofi Genzyme, Teva-Handok, and UCB; is a co-editor for the
Figures



References
-
- Jarius S, Ruprecht K, Kleiter I, et al. . MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13(1):280. doi:10.1186/s12974-016-0718-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
