Actionable mutations in early-stage ovarian cancer according to the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT): a descriptive analysis on a large prospective cohort
- PMID: 39705839
- PMCID: PMC11730936
- DOI: 10.1016/j.esmoop.2024.104090
Actionable mutations in early-stage ovarian cancer according to the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT): a descriptive analysis on a large prospective cohort
Abstract
Background: According to the European Society for Clinical Oncology (ESMO) guidelines, the therapeutic algorithm for early-stage epithelial ovarian carcinoma (EOC) is primarily based on grading and histotype. Adjuvant chemotherapy is usually recommended for high-grade tumors and for the International Federation of Gynecology and Obstetrics (FIGO) stage IB-IC; however, overtreatment remains a concern. Conversely, patients truly at higher risk of recurrence currently lack access to additional therapeutic strategies.
Patients and methods: This study presents a descriptive analysis of early-stage EOC patients who were prospectively sequenced and stratified into high-, intermediate-, and low-risk groups based on clinicopathological features. Oncogenic alterations were identified using OncoKB and classified according to the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT) Tier I-III. The prevalence of molecular findings was first reported for each risk subgroup, followed by an analysis on the cohort of patients who experienced relapse.
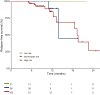
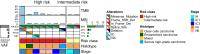
Results: A total of 180 patients with FIGO stage I-II EOC were enrolled between January 2022 and December 2023; 126 patients (70%) had at least one ESCAT Tier I-III alteration (including 51% high risk, 35% intermediate risk, and 14% low risk); among them, approximately one-quarter (26%, 95% confidence interval 19% to 35%) had an ESCAT Tier I alteration. BRCA1 and BRCA2 alterations were observed in about one-quarter of patients, with BRCA2 often co-altered with POLE mutations (55%, P = 2.1 × 10-4). Notably, almost all BRCA1 variants were found in high-risk patients. BRAF V600E mutation (ESCAT IC) was found in 2.4% of patients. PIK3CA variants were the most common Tier IIIA alterations found in 59% of patients. Among those who experienced recurrence, 60% had at least one ESCAT Tier I-III alteration, with PIK3CA mutations being the most frequent.
Conclusions: These findings highlight the potential for actionable alterations in most early-stage EOC patients and support the exploration of chemotherapy-free regimens for low- to intermediate-risk groups, as well as targeted maintenance therapy for high-risk individuals.
Keywords: ESCAT; actionable alterations; early-stage ovarian cancer; target therapy.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Ledermann J.A., Matias-Guiu X., Amant F., et al. ESGO-ESMO-ESP consensus conference recommendations on ovarian cancer: pathology and molecular biology and early, advanced and recurrent disease. Ann Oncol. 2024;35(3):248–266. - PubMed
-
- Trimbos J.B., Parmar M., Vergote I., et al. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst. 2003;95(2):105–112. - PubMed
-
- Kleppe M., van der Aa M.A., Van Gorp T., Slangen B.F., Kruitwagen R.F. The impact of lymph node dissection and adjuvant chemotherapy on survival: a nationwide cohort study of patients with clinical early-stage ovarian cancer. Eur J Cancer. 2016;66:83–90. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

