Inhibiting de novo lipogenesis identifies a therapeutic vulnerability in therapy-resistant colorectal cancer
- PMID: 39705849
- PMCID: PMC11729006
- DOI: 10.1016/j.redox.2024.103458
Inhibiting de novo lipogenesis identifies a therapeutic vulnerability in therapy-resistant colorectal cancer
Abstract
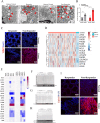
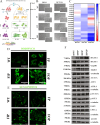
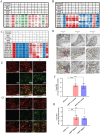
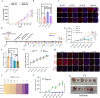
A significant clinical challenge in patients with colorectal cancer (CRC), which adversely impacts patient survival, is the development of therapy resistance leading to a relapse. Therapy resistance and relapse in CRC is associated with the formation of lipid droplets (LD) by stimulating de novo lipogenesis (DNL). However, the molecular mechanisms underlying the increase in DNL and the susceptibility to DNL-targeted therapies remain unclear. Our study demonstrates that colorectal drug-tolerant persister cells (DTPs) over-express Lipin1 (LPIN1), which facilitates the sequestration of free fatty acids into LDs. The increased expression is mediated by the ETS1-PTPN1-c-Src-CEBPβ pathway. Blocking the conversion of free fatty acids into LDs by treatment with statins or inhibiting lipin1 expression disrupts lipid homeostasis, leading to lipotoxicity and ferroptotic cell death in both DTPs and patient-derived organoids (PDOs) in vitro. Ferroptosis inhibitors or N-acetylcysteine (NAC) can alleviate lipid ROS and cell death resulting from lipin1 inhibition. This strategy also significantly reduces tumor growth in CRC DTP mouse xenograft and patient-derived xenograft (PDX) models. Our findings highlight a new metabolic vulnerability in CRC DTPs, PDO, and PDX models and provide a framework for the rational repurposing of statins. Targeting the phosphatidic acid (PA) to diacylglycerol (DAG) conversion to prevent lipid droplet formation could be an effective therapeutic approach for therapy-resistant CRC.
Keywords: De novo lipogenesis; Drug tolerant persister cells; Ferroptosis; Lipid droplet; Lipin1; Non-responder.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Briffa R., et al. Colorectal Cancer-Diagnosis. Screening and Management; 2017. Acquired and intrinsic resistance to colorectal cancer treatment; pp. 57–81.
-
- Vallam K.C., et al. Adenocarcinoma of the rectum—a composite of three different subtypes with varying outcomes? Clin. Colorectal Cancer. 2016;15(2):e47–e52. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

