Exploiting O-GlcNAc dyshomeostasis to screen O-GlcNAc transferase intellectual disability variants
- PMID: 39706180
- PMCID: PMC11784489
- DOI: 10.1016/j.stemcr.2024.11.010
Exploiting O-GlcNAc dyshomeostasis to screen O-GlcNAc transferase intellectual disability variants
Abstract
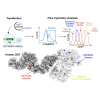
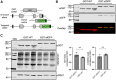
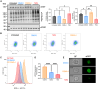
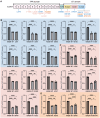
O-GlcNAcylation is an essential protein modification catalyzed by O-GlcNAc transferase (OGT). Missense variants in OGT are linked to a novel intellectual disability syndrome known as OGT congenital disorder of glycosylation (OGT-CDG). The mechanisms by which OGT missense variants lead to this heterogeneous syndrome are not understood, and no unified method exists for dissecting pathogenic from non-pathogenic variants. Here, we develop a double-fluorescence strategy in mouse embryonic stem cells to measure disruption of O-GlcNAc homeostasis by quantifying the effects of variants on endogenous OGT expression. OGT-CDG variants generally elicited a lower feedback response than wild-type and Genome Aggregation Database (gnomAD) OGT variants. This approach was then used to dissect new putative OGT-CDG variants from pathogenic background variants in other disease-associated genes. Our work enables the prediction of pathogenicity for rapidly emerging de novo OGT-CDG variants and points to reduced disruption of O-GlcNAc homeostasis as a common mechanism underpinning OGT-CDG.
Keywords: O-GlcNAc; OGT; OGT-CDG; neurodevelopment.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

