Global impact of ten-valent and 13-valent pneumococcal conjugate vaccines on invasive pneumococcal disease in all ages (the PSERENADE project): a global surveillance analysis
- PMID: 39706204
- PMCID: PMC11947069
- DOI: 10.1016/S1473-3099(24)00665-0
Global impact of ten-valent and 13-valent pneumococcal conjugate vaccines on invasive pneumococcal disease in all ages (the PSERENADE project): a global surveillance analysis
Erratum in
-
Correction to Lancet Infect Dis 2024; published online Dec 17. https://doi.org/10.1016/S1473-3099(24)00665-0.Lancet Infect Dis. 2025 Mar;25(3):e137. doi: 10.1016/S1473-3099(25)00032-5. Epub 2025 Jan 17. Lancet Infect Dis. 2025. PMID: 39832512 Free PMC article. No abstract available.
Abstract
Background: Pneumococcal conjugate vaccines (PCVs) that are ten-valent (PCV10) and 13-valent (PCV13) became available in 2010. We evaluated their global impact on invasive pneumococcal disease (IPD) incidence in all ages.
Methods: Serotype-specific IPD cases and population denominators were obtained directly from surveillance sites using PCV10 or PCV13 in their national immunisation programmes and with a primary series uptake of at least 50%. Annual incidence rate ratios (IRRs) were estimated comparing the incidence before any PCV with each year post-PCV10 or post-PCV13 introduction using Bayesian multi-level, mixed-effects Poisson regressions, by site and age group. All site-weighted average IRRs were estimated using linear mixed-effects regression, stratified by product and previous seven-valent PCV (PCV7) effect (none, moderate, or substantial).
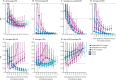
Findings: Analyses included 32 PCV13 sites (488 758 cases) and 15 PCV10 sites (46 386 cases) in 30 countries, primarily high income (39 sites), using booster dose schedules (41 sites). By 6 years after PCV10 or PCV13 introduction, IPD due to PCV10-type serotypes and PCV10-related serotype 6A declined substantially for both products (age <5 years: 83-99% decline; ≥65 years: 54-96% decline). PCV7-related serotype 19A increases before PCV10 or PCV13 introduction were reversed at PCV13 sites (age <5 years: 61-79% decline relative to before any PCV; age ≥65 years: 7-26% decline) but increased at PCV10 sites (age <5 years: 1·6-2·3-fold; age ≥65 years: 3·6-4·9-fold). Serotype 3 IRRs had no consistent trends for either product or age group. Non-PCV13-type IPD increased similarly for both products (age <5 years: 2·3-3·3-fold; age ≥65 years: 1·7-2·3-fold). Despite different serotype 19A trends, all-serotype IPD declined similarly between products among children younger than 5 years (58-74%); among adults aged 65 years or older, declines were greater at PCV13 (25-29%) than PCV10 (4-14%) sites, but other differences between sites precluded attribution to product.
Interpretation: Long-term use of PCV10 or PCV13 reduced IPD substantially in young children and more moderately in older ages. Non-vaccine-type serotypes increased approximately two-fold to three-fold by 6 years after introduction of PCV10 or PCV13. Continuing serotype 19A increases at PCV10 sites and declines at PCV13 sites suggest that PCV13 use would further reduce IPD at PCV10 sites.
Funding: Bill & Melinda Gates Foundation as part of the WHO Pneumococcal Vaccines Technical Coordination Project.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests KH reports employment at Pfizer from Oct 26, 2020. MDK reports grants from Merck and Pfizer, and personal fees from Merck. JAS reports grants from the Bill & Melinda Gates Foundation, the Wellcome Trust, the UK Medical Research Council, and the National Institute of Health Research. M-CCB reports lecture fees from MSD. ASk reports grants and personal fees from MSD and Pfizer. MvdL reports support from, membership on advisory boards for, and speakers honoraria from Pfizer and Merck. SD reports a grant from Pfizer. KA reports a grant from Merck. AvG reports research funding from Pfizer, and attendance at advisory board meetings for Pfizer and Merck. AM reports research support to her institution from Pfizer and Merck, and honoraria for advisory board membership from GSK, Merck, and Pfizer. SNL performs contract research for GSK, Pfizer, and Sanofi Pasteur on behalf of St George's University of London, with no personal remuneration. IY reports membership of an mRNA-1273 study group, and funding to her institution to conduct clinical research from BioFire, MedImmune, Regeneron, PaxVax, Pfizer, GSK, Merck, Novavax, Sanofi Pasteur, and Micron. RD reports grants or research support from Pfizer, MSD, and Medimmune; scientific consultancy for Pfizer, MeMed, MSD, and BiondVax; participation on advisory boards of Pfizer, MSD, and BiondVax; and being a speaker for Pfizer. LLH reports research grants to her institution from GSK, Pfizer, and Merck. JK reports an unrestricted grant-in-aid from Pfizer Canada. MHi reports reimbursement for advisory boards from MSD; and an investigator-initiated research grant from Pfizer paid to his institution. JCS reports assistance from Pfizer for attending scientific meetings. NPK reports research support from Pfizer, GSK, Sanofi Pasteur, Merck, and Protein Sciences (now Sanofi Pasteur). CC reports research support to her institution from Sanofi Pasteur. KGK reports grants from GSK. All other authors declare no competing interests.
Figures



References
-
- Troeger C, Blacker B, Khalil IA, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–1210. - PMC - PubMed
-
- WHO Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly Epidemiol Rec. 2007;82:93–104. - PubMed
-
- View-hub by IVAC Pneumococcal conjugate vaccine (PCV) https://view-hub.org/vaccine/pcv
-
- Deloria Knoll M, Bennett JC, Garcia Quesada M, et al. Global landscape review of serotype-specific invasive pneumococcal disease surveillance among countries using PCV10/13: the Pneumococcal Serotype Replacement and Distribution Estimation (PSERENADE) project. Microorganisms. 2021;9:742. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

