The physicochemical properties of lipopolysaccharide chemotypes regulate activation of the contact pathway of blood coagulation
- PMID: 39706265
- PMCID: PMC11773025
- DOI: 10.1016/j.jbc.2024.108110
The physicochemical properties of lipopolysaccharide chemotypes regulate activation of the contact pathway of blood coagulation
Abstract
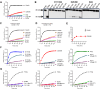
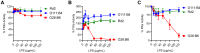
Lipopolysaccharide (LPS) is the primary pathogenic factor in Gram-negative sepsis. While the presence of LPS in the bloodstream during infection is associated with disseminated intravascular coagulation, the mechanistic link between LPS and blood coagulation activation remains ill-defined. The contact pathway of coagulation-a series of biochemical reactions that initiates blood clotting when plasma factors XII (FXII) and XI (FXI), prekallikrein (PK), and high molecular weight kininogen interact with anionic surfaces-has been shown to be activated in Gram-negative septic patients. In this study, using an in vivo baboon model of Gram-negative Escherichia coli sepsis, we observed activation of the contact pathway including FXII, FXI, and PK. We examined whether E.coli LPS molecules could bind and activate contact pathway members by quantifying the interaction and activation of either FXII, FXI, or PK with each of the three chemotypes of LPS: O111:B4, O26:B6, or Rd2. The LPS chemotypes exhibited distinct physicochemical properties as aggregates and formed complexes with FXII, FXI, and PK. The LPS chemotype O26:B6 uniquely promoted the autoactivation of FXII to FXIIa and, in complex with FXIIa, promoted the cleavage of FXI and PK to generate FXIa and plasma kallikrein, respectively. Furthermore, in complex with the active forms of FXI or PK, LPS chemotypes were able to regulate the catalytic activity of FXIa and plasma kallikrein, respectively, despite the inability to promote the autoactivation of either zymogen. These data suggest that the procoagulant phenotype of E.coli is influenced by bacterial strain and the physicochemical properties of the LPS chemotypes.
Keywords: coagulation; contact pathway; factor XII; lipopolysaccharides; sepsis.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest C. U. L. and E. I. T. are employees of Aronora, Inc, a company that may have a commercial interest in the results of this research. J. J. S. serves as a medical consultant for Aronora, Inc. This potential conflict of interest has been reviewed and managed by conflict of interest in research committee of the Oregon Health & Science University. The remaining authors declare that they have no conflicts of interest with the contents of this article.
Figures








Similar articles
-
Immobilized transition metal ions stimulate contact activation and drive factor XII-mediated coagulation.J Thromb Haemost. 2012 Oct;10(10):2108-15. doi: 10.1111/j.1538-7836.2012.04890.x. J Thromb Haemost. 2012. PMID: 22905925 Free PMC article.
-
Factor XII promotes blood coagulation independent of factor XI in the presence of long-chain polyphosphates.J Thromb Haemost. 2013 Jul;11(7):1341-52. doi: 10.1111/jth.12295. J Thromb Haemost. 2013. PMID: 23659638 Free PMC article.
-
Proteolytic activity of contact factor zymogens.J Thromb Haemost. 2021 Feb;19(2):330-341. doi: 10.1111/jth.15149. Epub 2020 Dec 7. J Thromb Haemost. 2021. PMID: 33107140 Free PMC article. Review.
-
Factor XII Activation Promotes Platelet Consumption in the Presence of Bacterial-Type Long-Chain Polyphosphate In Vitro and In Vivo.Arterioscler Thromb Vasc Biol. 2018 Aug;38(8):1748-1760. doi: 10.1161/ATVBAHA.118.311193. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354195 Free PMC article.
-
Factor XII in coagulation, inflammation and beyond.Cell Signal. 2018 Nov;51:257-265. doi: 10.1016/j.cellsig.2018.08.006. Epub 2018 Aug 15. Cell Signal. 2018. PMID: 30118759 Review.
Cited by
-
Cellular and Molecular Mechanisms Explaining the Link Between Inflammatory Bowel Disease and Heart Failure.Cells. 2025 Jul 21;14(14):1124. doi: 10.3390/cells14141124. Cells. 2025. PMID: 40710377 Free PMC article. Review.
-
Lipopolysaccharide supramolecular organization regulates the activation of coagulation factor XII.Biochim Biophys Acta Biomembr. 2025 Mar;1867(3):184415. doi: 10.1016/j.bbamem.2025.184415. Epub 2025 Feb 6. Biochim Biophys Acta Biomembr. 2025. PMID: 39922445
References
-
- Kellum J.A., Formeck C.L., Kernan K.F., Gómez H., Carcillo J.A. Subtypes and mimics of sepsis. Crit. Care Clin. 2022;38:195–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

