Targeting PTGDS Promotes ferroptosis in peripheral T cell lymphoma through regulating HMOX1-mediated iron metabolism
- PMID: 39706989
- PMCID: PMC11833084
- DOI: 10.1038/s41416-024-02919-w
Targeting PTGDS Promotes ferroptosis in peripheral T cell lymphoma through regulating HMOX1-mediated iron metabolism
Abstract
Background: Peripheral T cell lymphoma (PTCL) is characterized by high heterogeneity, strong aggressiveness, and extremely poor prognosis. Ferroptosis, a novel form of programmed cell death, has been involved in tumor development and targeting ferroptosis holds great potential for tumor therapy.
Methods: Lentiviral transfection was performed to regulate gene expression, followed by Tandem mass tag (TMT)-mass spectrometry and RNA-sequencing. Tumor xenograft models were established for in vivo experiments.
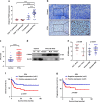
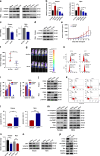
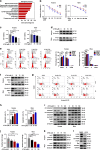
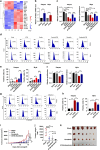
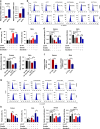
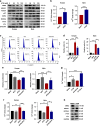
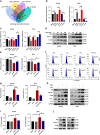
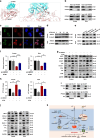
Results: High expression of prostaglandin D2 synthase (PTGDS) was closely associated with poor prognosis of PTCL patients. PTGDS knockdown and AT56 treatment significantly inhibited the progression of PTCL through regulating cell viability, proliferation, apoptosis, cell cycle and invasion in vitro and in vivo. We further revealed that targeting PTGDS promoted ferroptosis process and enhanced the sensitivity of PTCL cells to ferroptosis inducers Sorafenib in vitro and in vivo. Mechanically, PTGDS interacted with heme-degrading enzymes HMOX1, and targeting PTGDS increased the level of iron and induced ferroptosis in PTCL through promoting HMOX1-mediated heme catabolism and ferritin autophagy process. Through the construction of H25A mutation, the specific gene site of HMOX1 corresponding to its role was identified.
Conclusions: Taken together, our findings firstly identified that targeting PTGDS promotes the ferroptosis in PTCL through regulating HMOX1-mediated iron metabolism, and highlighted novel therapeutic strategies to improve the efficacy of ferroptosis-targeted therapy in PTCL patients.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This study was approved by the Medical Ethical Committee of Shandong Provincial Hospital (SZRJJ:NO.2022-123) and written informed consent in accordance with the Declaration of Helsinki was obtained from each patient. All animal experiments were approved by Animal Care and Research Advisory Committee of Shandong Provincial Hospital (NO.SD NSFC 2023-0185). All methods were performed in accordance with its relevant guidelines and regulations.
Figures
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: a cancer J Clin. 2023;73:17–48. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

