miR-21-loaded bone marrow mesenchymal stem cell-derived exosomes inhibit pyroptosis by targeting MALT1 to repair chemotherapy-induced premature ovarian insufficiency
- PMID: 39707056
- PMCID: PMC11662076
- DOI: 10.1007/s10565-024-09946-6
miR-21-loaded bone marrow mesenchymal stem cell-derived exosomes inhibit pyroptosis by targeting MALT1 to repair chemotherapy-induced premature ovarian insufficiency
Abstract
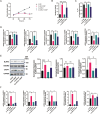
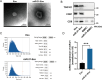
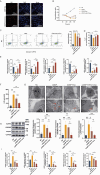
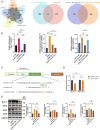
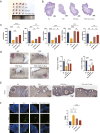
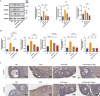
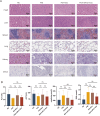
Chemotherapy is essential for treating malignant tumors, but it can cause premature ovarian insufficiency (POI). Recent studies suggest that exosomes enriched with miR-21 (miR-21-Exo) may help mitigate POI, though the underlying mechanisms remain largely unexplored. This research investigates how miR-21-Exo influences chemotherapy-induced POI using an experimental model where KGN cells are exposed to cisplatin. We assessed the impact of miR-21 on cellular activity and generated miR-21 overexpressing bone marrow mesenchymal stem cells (miR-21-BMSC) via lentiviral modification. Isolated miR-21-Exo was analyzed for its effects on cellular function. Bioinformatics identified Mucosa-Associated Lymphoid Tissue Lymphoma Translocation Protein 1 (MALT1) as a target of miR-21. We confirmed that miR-21-Exo regulates MALT1 and the NF-κB signaling pathway to prevent cell pyroptosis. Further studies in a rat model demonstrated the therapeutic potential and safety of miR-21-Exo. Overall, our findings highlight a novel strategy for addressing chemotherapy-induced POI by modulating MALT1 and the NF-κB pathway, offering significant therapeutic implications.
Keywords: Bone mesenchymal stem cell; Exosome; MALT1; MiR-21; Premature ovarian insufficiency; Pyroptosis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and informed consent: All animal experiments conducted was compliant with the Ethics Committee of Southern Medical University Zhujiang Hospital (Nọ: LAEC-2022–208). Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Cai JH, Sun YT, Bao S. Hucmscs-exosomes containing mir-21 promoted estrogen production in ovarian granulosa cells via lats1-mediated phosphorylation of loxl2 and yap. Gen Comp Endocrinol. 2022;321–322:114015. 10.1016/j.ygcen.2022.114015. - PubMed
-
- Ding C, Zhu L, Shen H, Lu J, Zou Q, HuangHuang CB. Exosomal mirna-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating sirt7. Stem Cells. 2020b;38(9):1137–48. 10.1002/stem.3204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

