Lung immune incompetency after mild peritoneal sepsis and its partial restoration by type 1 interferon: a mouse model study
- PMID: 39707074
- PMCID: PMC11662124
- DOI: 10.1186/s40635-024-00707-7
Lung immune incompetency after mild peritoneal sepsis and its partial restoration by type 1 interferon: a mouse model study
Abstract
Background: Sepsis is commonly associated with acute respiratory distress syndrome (ARDS). Although the exaggerated inflammation may damage intact lung tissues, a percentage of patients with ARDS are reportedly immunocompromised, with worse outcomes. Herein, using a murine sepsis model, time-course immune reprogramming after sepsis was evaluated to explore whether the host is immunocompromised. Leukocyte kinetics in the lung tissue were evaluated in a male C57/BL6 mouse model of mild peritoneal sepsis induced by cecal ligation and puncture, with the survival rate exceeds 90%. Lung immune reactivity was evaluated by intratracheal instillation of lipopolysaccharide (LPS; 30 µg). Furthermore, the effect of interferon (IFN)-β in vivo and ex vivo was evaluated.
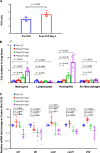
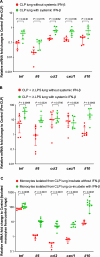
Results: Four days after sepsis, the lung water content remained high, even among mice in clinical recovery. While monocytes and neutrophils gradually accumulated in the lung interstitium, the inflammatory cytokine/chemokine expression levels in the lungs continued to decline. Intratracheal LPS instillation induced more leukocyte trafficking and protein leakage into the alveoli in the septic lung, indicating more severe lung injury. However, LPS stimulation-associated mRNA expression of tnf, il6, ccl2, and cxcl1 was suppressed. Intra-alveolar expression of tumor necrosis factor (TNF)-α, interleukin (IL)-6, monocyte chemoattractant protein (MCP)-1, and keratinocyte-derived cytokine (KC) was also suppressed. Monocytes isolated from the lung tissue showed an impaired response in il6, ccl2, and cxcl1 to LPS. Systemic IFN-β restored the above impaired regulator function of monocytes, as did coculturing these cells from lung tissue with IFN-β.
Conclusions: Histologically accelerated inflammation and paradoxically suppressed immunological regulator signaling were observed in the early recovery phase of sepsis. This observation may provide a model for the immunologically irresponsive state that occurs in some patients with sepsis. Systemic IFN-β partly restored the post-septic immunocompromised state, indicating its therapeutic potential for the immunosuppressive state seen in some patients with sepsis/ARDS.
Keywords: ARDS; Acute lung injury; Immune reprogramming; Immune suppression; Innate immunity; Interferon-β; Sepsis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The experimental protocols used for all animal experiments were approved by the University of Tokyo Graduate School of Medicine Institutional Review Board (#Med-P20-106), conformed to the NIH Guide for the Care and Use of Laboratory Animals (1985 revision), and were conducted with strict compliance with ethical guidelines issued by the University of Tokyo Graduate School of Medicine. Male C57/BL6 mice (Jackson Laboratories, Clea Japan, Tokyo, Japan), 8–10 weeks of age and 20–25 g in body weight, were purchased for use in animal experiments. Consent for publication: Not applicable. Competing interests: Uchida K is in collaboration with Nihon Kohden Corporation and Nipro Corporation. Neither of these collaborations have any relationship with the present study. No conflicts of interest are declared by any other authors.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

