A New Perspective on Drugs for Duchenne Muscular Dystrophy: Proposals for Better Respiratory Outcomes and Improved Regulatory Pathways
- PMID: 39707120
- PMCID: PMC11829838
- DOI: 10.1007/s40272-024-00673-3
A New Perspective on Drugs for Duchenne Muscular Dystrophy: Proposals for Better Respiratory Outcomes and Improved Regulatory Pathways
Abstract
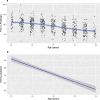
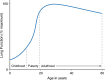
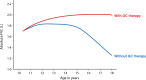
New drugs for Duchenne muscular dystrophy (DMD) are emerging rapidly. However, we and others believe these drugs are achieving regulatory approval prematurely. It is the cardiorespiratory complications of DMD that cause the disease's major morbidities and that determine survival. Thus, to be truly effective, a new drug must improve cardiorespiratory function; instead, new drugs are approved for patient use via accelerated regulatory pathways that rely on surrogate outcome measures with unproven clinical benefits (such as tissue levels of non-biologic, truncated dystrophin) and on scales that reflect muscle strength (such as small improvements in timed activities). In DMD, cardiorespiratory complications occur in "older" individuals who are in the non-ambulatory stage of the disease. In contrast, accelerated approvals are based on data from young, ambulatory subjects, a group that essentially never experiences cardiorespiratory complications. When drug studies do obtain cardiorespiratory data, their methodologies are suboptimal. We critically review these methodologies in detail, including problems with the use of threshold levels of respiratory function as outcome measures; problems with the use of historical controls, whose results vary widely, and are influenced by uncontrolled variables related to their observational nature; and the limitations of using percent predicted forced vital capacity (FVC %pred), and its single rate of decline across a wide range of age and function, as a preferred respiratory outcome measure. We discuss the advantages of an alternative respiratory outcome, the absolute value of FVC with aging (the "Rideau plot"). Unlike FVC %pred, the Rideau plot considers distinct phenotypes rather than aggregating all individuals into a single respiratory trajectory. Key features of the Rideau plot can show the nature and timing of a drug's effect on respiratory function, making it a potentially better outcome measure for assessing the respiratory effects of a drug. With this article, we use our respiratory perspective to critically examine the DMD drug development process and to propose improvements in study methodologies and in the regulatory processes that approve new drugs.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Funding: No funding was received for the writing of this article. Conflict of interest: DJB reports a financial interest in granted (US patents 8,651,107; 8,844,530; 9,795,752; and 10,814,082, and related international patents) and pending patents for respiratory devices, licensed to Advanced Bio Machines PTE (ABM Respiratory Care). DWS reports reimbursement for travel expenses to a conference convened by the patient advocacy group Parent Project MD. JBB, HMB, MLD, and SLK declare no competing interests. Availability of data and material: The data analyzed during this study are included in this published article (see Fig. 1). Ethics approval: In Sect. 3.3 of this article, previously published data from the CNDR were re-analyzed. Patients are recruited to the CNDR in accordance with local ethics approval processes (see: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7902956/ ). Requests for research projects are approved by the CNDR advisory committee. For this analysis, de-identified registry data were shared with permission. Consent to participate: Not applicable. Consent for publication:: Not applicable. Code availability:: Not applicable. Author contributions: DJB was responsible for the conceptualization and initial drafts of this article. All authors contributed to the literature search, data analysis, methodology, and review and revision of the manuscript. All authors reviewed and approved the final manuscript before submission.
Figures


References
-
- Conway KM, Thomas S, Ciafaloni E, et al. Prophylactic use of cardiac medications for delay of left ventricular dysfunction in Duchenne muscular dystrophy. Birth Defects Res. 2023. 10.1002/bdr2.2260. - PubMed
-
- Boussaid G, Stalens C, Devaux C, Segovia-Kueny S, Lofaso F, Reveillere C. Impact of mechanical ventilation methods on the life perception of subjects with Duchenne muscular dystrophy: French cross-sectional survey. Respir Care. 2020;65:1712–20. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

