Endothelial-specific deletion of connexin 43 improves renal function and structure after acute kidney injury
- PMID: 39707203
- PMCID: PMC11660768
- DOI: 10.1186/s10020-024-01011-6
Endothelial-specific deletion of connexin 43 improves renal function and structure after acute kidney injury
Abstract
Background: We have previously reported that the gap junction protein connexin 43 (Cx43) was upregulated in chronic renal disease in humans and rodents and plays a crucial role in the progression of experimental nephropathy. In this study, we investigated its role after renal ischemia/reperfusion (rIR), which is a major mechanism of injury in acute renal injury (AKI) and renal transplant graft dysfunction.
Methods: Wild-type mice (WT) and mice in which Cx43 expression was genetically reduced by half (Cx43 ±) were unilaterally nephrectomized. The left renal artery was subsequently clamped, with reperfusion of varying duration. Mice with tubular- or endothelial-specific deletion of Cx43 were also used to assess the effect of this connexin in each cell type after rIR. Kidneys were assessed for histological evaluation, immunohistochemistry, and RT-PCR.
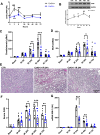
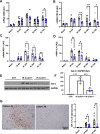
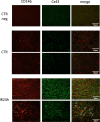
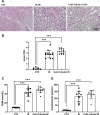
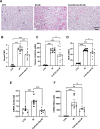
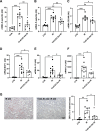
Results: Blood urea nitrogen and creatininemia were progressively elevated in WT mice and picked up 48 h after rIR. At the same time point, severe tubular necrosis and dilation occurred in the cortico-medullary junction of the injured kidneys with accompanying massive neutrophil infiltration. Interestingly, Cx43 expression was progressively increased within the tubulointerstitial compartment during kidney damage progression and was paralleled closely by that of markers of renal dysfunction. Cx43 ± mice showed fewer tubular lesions, less inflammation, and further improved renal function. Similar results were observed in mice where Cx43 was specifically deleted within the vascular endothelium. In contrast, Cx43 deletion in renal tubules did not significantly improve renal structure and function after rIR.
Conclusion: Our findings suggest that endothelial Cx43 plays a crucial role in AKI.
Keywords: Acute kidney injury; Connexin 43; Inflammation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Written informed consent was provided by all participants. All animal protocols were approved by the appropriate committee of the National Institute for Health and Medical Research (Inserm) and the Sorbonne University (Paris, France). Consent for publication: Not applicable. Competing interests: The authors have declared that no conflict of interest exists.
Figures

References
-
- Abed A, Toubas J, Kavvadas P, Authier F, Cathelin D, Alfieri C, Boffa JJ, Dussaule JC, Chatziantoniou C, Chadjichristos CE. Targeting connexin 43 protects against the progression of experimental chronic kidney disease in mice. Kidney Int. 2014;86:768–79. - PubMed
-
- Abed A, Leroyer AS, Kavvadas P, Authier F, Bachelier R, Foucault-Bertaud A, Bardin N, Cohen CD, Lindenmeyer MT, Genest M, Joshkon A, Jourde-Chiche N, Burtey S, Blot-Chabaud M, Dignat-George F, Chadjichristos CE. Endothelial-specific deletion of CD146 protects against experimental glomerulonephritis in mice. Hypertension. 2021;77(4):1260–72. - PubMed
-
- Bardin N, Reumaux D, Geboes K, Colombel JF, Blot-Chabaud M, Sampol J, Duthilleul P, Dignat-George F. Increased expression of CD146, a new marker of the endothelial junction in active inflammatory bowel disease. Inflamm Bowel Dis. 2006;12(1):16–21. - PubMed
-
- Bardin N, Blot-Chabaud M, Despoix N, Kebir A, Harhouri K, Arsanto JP, Espinosa L, Perrin P, Robert S, Vely F, Sabatier F, Le Bivic A, Kaplanski G, Sampol J, Dignat-George F. CD146 and its soluble form regulate monocyte transendothelial migration. Arterioscler Thromb Vasc Biol. 2009;29(5):746–53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

