ABL1‒YAP1 axis in intestinal stem cell activated by deoxycholic acid contributes to hepatic steatosis
- PMID: 39707364
- PMCID: PMC11662811
- DOI: 10.1186/s12967-024-05865-6
ABL1‒YAP1 axis in intestinal stem cell activated by deoxycholic acid contributes to hepatic steatosis
Abstract
Background: Yes-associated protein 1 (YAP1) regulates the survival, proliferation, and stemness of cells, and contributes to the development of metabolic dysfunction associated fatty liver disease (MAFLD). However, the regulatory role of intestinal YAP1 in MAFLD still remains unclear.
Methods: Terminal ileal specimens were used to compare intestinal YAP1 activation in patients with and without MAFLD. Mice targeted for knocking out YAP1 in the intestinal epithelium were fed a high-fat diet (HFD) for 8 consecutive weeks. In a separate group, the mice were fed an HFD supplemented with the bile acid binder cholestyramine (CHO) or a low-fat diet with deoxycholic acid (DCA). Immunofluorescence, Immunohistochemistry, Western blot, RT-qPCR, ELISA, 16S rDNA sequencing, tissue and enteroid culture techniques were used to evaluate the effects of an HFD or DCA on the gut‒liver axis in mice or humans.
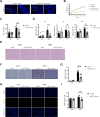
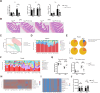
Results: Intestinal YAP1 was activated in both humans with MAFLD and mice fed an HFD. In in vivo studies, YAP1 knockout in intestinal epithelial cells of mice alleviated the hepatic steatosis induced by an HFD, and mitigated the adverse effects of HFD on the gut‒liver axis, including the upregulation of lipopolysaccharide (LPS) and inflammation levels, enrichment of intestinal Gram-negative bacteria, and inhibition of intestinal stem cell (ISC) differentiation into the goblet and Paneth cells. High-fat feeding (HFF) produced high concentrations of DCA. The consumption of DCA mimics these HFF-induced changes, and is accompanied by the activation of Abelson tyrosine-protein kinase 1 (ABL1) and its direct substrate, YAP1, in the terminal ileum. In vitro studies further confirmed that DCA upregulated the tyrosine phosphorylation of YAP1Y357 in ISC by activating ABL1, which inhibited the differentiation of ISCs into secretory cells.
Conclusions: Our findings reveal that the activation of the ABL1‒YAP1 axis in ISCs by DCA contributes to hepatic steatosis through the gut‒liver axis, which may provide a potential intestinal therapeutic target for MAFLD.
Keywords: Deoxycholic acid; Hepatic steatosis; High-fat diet; Intestinal stem cell; Yes-associated protein 1.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal procedures were approved by the Institutional Animal Care and Use Committee of the Shanghai General Hospital (protocol code 2021SQ205, 1 Match 2021). The human study was approved by the Ethics Committee of the Shanghai General Hospital (approval ID 20240226025226875). The study protocol adhered to the ethical principles of medical research involving human subjects as outlined in the 2013 Helsinki Declaration and the 2018 Istanbul Declaration. Consent for publication: Not applicable. Competing interests: The authors declare that they have no conflict of interest or financial conflicts to disclose.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

