Targeted lipid nanoparticles distributed in hydrogel treat osteoarthritis by modulating cholesterol metabolism and promoting endogenous cartilage regeneration
- PMID: 39707367
- PMCID: PMC11662830
- DOI: 10.1186/s12951-024-02965-9
Targeted lipid nanoparticles distributed in hydrogel treat osteoarthritis by modulating cholesterol metabolism and promoting endogenous cartilage regeneration
Abstract
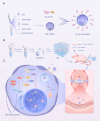
Osteoarthritis (OA) is the most common disease in aging joints and has characteristics of cartilage destruction and inflammation. It is currently considered a metabolic disease, and the CH25H-CYP7B1-RORα axis of cholesterol metabolism in chondrocytes plays a crucial catabolic regulatory role in its pathogenesis. Targeting of this axis in chondrocytes may provide a therapeutic approach for OA treatment. Here, in this study, we propose to use a combination of stem cell-recruiting hydrogels and lipid nanoparticles (LNPs) that modulate cholesterol metabolism to jointly promote a regenerative microenvironment. Specifically, we first developed an injectable, bioactive hydrogel composed of self-assembling peptide nanofibers that recruits endogenous synovial stem cells (SMSCs) and promotes their chondrogenic differentiation. At the same time, LNPs that regulate cholesterol metabolism are incorporated into the hydrogel and slowly released, thereby improving the inflammatory environment of OA. Enhancements were noted in the inflammatory conditions associated with OA, alongside the successful attraction of mesenchymal stem cells (MSCs) from the synovial membrane. These cells were then observed to differentiate into chondrocytes, contributing to effective cartilage restoration and chondrocyte regeneration, thereby offering a promising approach for OA treatment. In summary, this approach provides a feasible siRNA-based therapeutic option, offering a potential nonsurgical solution for treatment of OA.
Keywords: Cartilage rejuvenation; Cholesterol metabolism; Hydrogel; Lipid nanoparticles; Osteoarthritis.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were conducted in compliance with the Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of Shandong Provincial Hospital (No.2023–004). Consent for publication: All the authors have approved the manuscript to publish. Competing interests: The authors declare no competing interests.
Figures








References
MeSH terms
Substances
Grants and funding
- 2020CXGC010502/Shandong Provincial Key Research and Development Program
- Grant No. 81672185/the National Natural Science Foundation of China
- Grant No. 81702152/the National Natural Science Foundation of China
- No. tsqn202211348/the Taishan Scholars Program for Young Experts of Shandong Province
- Grant No.ZR2022MH222/the Natural Science Foundation of Shandong Province
LinkOut - more resources
Full Text Sources
Medical

