Ubiquitin-specific protease 7 maintains c-Myc stability to support pancreatic cancer glycolysis and tumor growth
- PMID: 39707401
- PMCID: PMC11662425
- DOI: 10.1186/s12967-024-05962-6
Ubiquitin-specific protease 7 maintains c-Myc stability to support pancreatic cancer glycolysis and tumor growth
Abstract
Background: The typical pathological feature of pancreatic ductal adenocarcinoma (PDAC) is a significant increase in stromal reaction, leading to a hypoxic and poorly vascularized tumor microenvironment. Tumor cells undergo metabolic reprogramming, such as the Warburg effect, yet the underlying mechanisms are not fully understood.
Methods: Interference and overexpression experiments were conducted to analyze the in vivo and in vitro effects of USP7 on the growth and glycolysis of tumor cells. Small-molecule inhibitors of USP7 and transgenic mouse models of PDAC were employed to assess the consequences of targeting USP7 in PDAC. The molecular mechanism underlying USP7-induced c-Myc stabilization was determined by RNA sequencing, co-IP and western blot analyses.
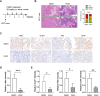
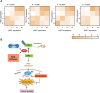
Results: USP7 is abnormally overexpressed in PDAC and predicts a poor prognosis. Hypoxia and extracellular matrix stiffness can induce USP7 expression in PDAC cells. Genetic silencing of USP7 inhibits the glycolytic phenotypes in PDAC cells, while its overexpression has the opposite effect, as demonstrated by glucose uptake, lactate production, and extracellular acidification rate. Importantly, USP7 promotes PDAC tumor growth in a glycolysis-dependent manner. The small-molecule inhibitor P5091 targeting USP7 effectively suppresses the Warburg effect and cell growth in PDAC. In a transgenic mouse model of PDAC, named KPC, P5091 effectively blocks tumor progression. Mechanistically, USP7 interacts with c-Myc, enhancing its stability and expression, which in turn upregulates expression of glycolysis-related genes.
Conclusions: This study sheds light on the molecular mechanisms underlying the Warburg effect in PDAC and unveils USP7 as a potential therapeutic target for improving PDAC treatment.
Keywords: Aerobic glycolysis; Deubiquitinating enzymes; Glucose metabolism; HAUSP.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Research Ethics Committee of Huashan Hospital, Fudan University and performed in accordance with the Declaration of Helsinki. All patients involved in this study signed informed consent. All the animal experiments in this study were approved by the Institutional Animal Care and Use Committee of Fudan university. Consent for publication: All authors agree to submit the article for publication. Competing interests: The authors declare that they have no competing interests.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

