Hymenoptera venom allergy in children
- PMID: 39707411
- PMCID: PMC11662473
- DOI: 10.1186/s13052-024-01731-9
Hymenoptera venom allergy in children
Abstract
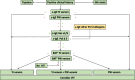
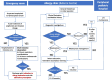
From a taxonomic point of view, Hymenoptera are subclassified into families: Apidae, including honeybees (Apis mellifera) and bumblebees (Bombus), and Vespidae, which, in turn, are divided into the subfamilies of Vespinae (wasps, including hornets, vespules, dolichovespules) and Polistinae (paper wasp). Hypersensitivity to Hymenoptera venom can be linked to immunological (IgE-mediated or non-IgE-mediated) and non-immunological mechanisms. Reactions are classified into local reactions, large local reactions, systemic reactions, toxic reactions, and unusual reactions. In general, children sensitize less frequently and have less severe reactions than adults, probably due to less exposure to repeated stings and fewer comorbidities. There are risk factors for systemic reactions that should be discussed with patients and their parents as appropriate. A correct diagnosis of Hymenoptera venom allergy relies on a careful clinical history and the appropriate use of skin and in vitro tests. The in vitro tests include serum specific IgE toward venom extracts and toward allergenic molecules. In complex diagnoses, CAP-inhibition and the Basophil Activation Test can also be used. In the presence of a systemic reaction, the basal serum tryptase measurement should be performed to rule out mastocytosis. In case of allergic reactions to Hymenoptera stings, in the acute phase, according to the current guidelines, the treatment of signs and symptoms mainly includes the use of adrenaline as first-line treatment in case of anaphylaxis and antihistamines and corticosteroids as subsequent lines of treatment. Given the impossibility of avoiding a new sting with certainty, the treatment of choice in subjects with hypersensitivity to Hymenoptera venom who have experienced systemic reactions is based on venom immunotherapy (VIT), with the venom of the responsible stinging insect identified after an adequate allergological work-up. VIT is performed in a suitable environment and has proved to be safe and effective with various administration protocols, both accelerated and conventional. The prevention of Hymenoptera venom anaphylaxis in patients who have already developed a previous episode is crucial and must be supported by environmental protection interventions and early therapy. Places where one is more likely to encounter insects and risky behaviors should be avoided.
Keywords: Allergy; Children; Hymenoptera; Pediatrics; Venom.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

