Postorgasmic illness syndrome benefiting from omalizumab and antidepressant: a case report
- PMID: 39707470
- PMCID: PMC11662807
- DOI: 10.1186/s13256-024-04986-2
Postorgasmic illness syndrome benefiting from omalizumab and antidepressant: a case report
Abstract
Background: Postorgasmic illness syndrome is characterized by flu, rhinitis, conjunctivitis, loss of appetite, muscle weakness, and fatigue after ejaculation, lasting 2-7 days. The multidisciplinary treatment approach, incorporating omalizumab and antidepressants, has rarely been documented in literature.
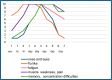
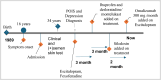
Case presentation: In this article, we present the case of a 33-year-old single Turkish male with postorgasmic illness syndrome, characterized by typical clinical symptoms and a positive autologous semen test. Notably, his serum estrogen and progesterone levels were elevated. The patient's Beck anxiety score was 42 points, the depression scale score was 37, and suicidal thoughts. Fresh autologous semen taken at the hospital was diluted with 0.9% saline, and prick and intradermal skin tests were performed.
Conclusion: The patient's symptoms improved significantly with the combination of omalizumab and escitalopram. This case not only provides a new perspective on the management of postorgasmic illness syndrome but also highlights the potential roles of allergic, psychiatric, and endocrinological mechanisms in the etiology and treatment of this complex condition.
Keywords: Allergy; Male orgasmic disorders; Postorgasm illness syndrome; Semen.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from the participant. The original article is not under consideration by another publication, and its substance, tables, or figures have not been published previously and will only be published elsewhere. Consent for publication: Written informed consent was obtained from the patient for publication of this case report and all accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. Competing interests: The authors declare that they have no competing interests.
Figures
References
-
- Waldinger MD, Schweitzer DH. Post-orgasmic illness syndrome: two cases. J Sex Marital Ther. 2002;28(3):251–5. - PubMed
-
- Depreux N, Basagaña M, Pascal M. Síndrome de enfermedad post-orgásmica: no evidencia de causalidad alérgica. Rev Int Androl. 2018;16(1):42–4. - PubMed
-
- Nguyen HMT, Bala A, Gabrielson AT, Hellstrom WJG. Post-orgasmic illness syndrome: a review. Sex Med Rev. 2018;6(1):11–5. 10.1016/j.sxmr.2017.08.006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources