Spinal muscular atrophy is also a disorder of spermatogenesis
- PMID: 39707482
- PMCID: PMC11662785
- DOI: 10.1186/s13023-024-03494-2
Spinal muscular atrophy is also a disorder of spermatogenesis
Abstract
Background: Spinal muscular atrophy (SMA) patients benefit from pre-mRNA splicing modifiers targeting the SMN2 gene, which aims to increase functional SMN production. The animal toxicity affecting spermatogenesis associated with one such treatment raised questions about male SMA patients' spermatogenesis.
Methods: This descriptive, cross-sectional study was conducted from June 2022 to July 2023. The study involved adult male patients with genetically confirmed SMA type 2 (SMA2) or SMA3 from 13 French neuromuscular centers. The patients' general data, motor severity, urological history, exposure to certain factors, parenthood, and spermogram results were obtained. All patients were enrolled prior to exposure to risdiplam.
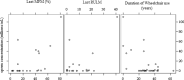
Findings: Sixty-eight patients were enrolled ( 36 SMA2 and 32 SMA3 patients). Forty-one patients had fertility data (parenthood history and spermogram analyses) and underwent 33 spermograms. Fertility disorders were identified in 27 of the 41 patients (65·9%, 95%CI 51·3-80·4%) in particular SMA2 patients: 19 cases (90.5%, CI 77·9-100%) (SMA3: 8 cases (40%, CI 18·5-61·5%). Among the patients with available spermograms, 81% (27/33) had abnormal sperm concentration; 30% presented azoospermia. These abnormalities were significantly associated with SMA type (more prevalent in SMA2 patients, p < 0·001), disease motor severity, which included age at the loss of walking ability and wheelchair use duration (p < 0·001). The Motor Function Measure (MFM) determined that the sperm counts were also correlated with disease severity (p < 0·01).
Interpretation: The fertility disorders were correlated with SMA severity and were particularly evident in SMA2 patients. In the latter, sperm concentration positively correlated with MFM. This study is the first one to link fertility disorders with spermogram abnormalities in SMA males. Understanding spermatogenesis in SMA is crucial, especially with new therapies such as risdiplam. Consequently, conducting systematic spermogram studies prior to SMA treatment is recommended.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The relevant French non-interventional research committee (GNEDS) approved the study. Consent for publication: Not Applicable. Competing interests: AM has received honoraria from lectures for Biogen. JBD, YP, ESC, PL and ADP have received honoraria from advisory board and lectures for Roche and Biogen. JBN has participated at scientific congress sponsoring from Biogen and Novartis. GS has received financial support from Roche and Biogen and participated at scientific congress sponsoring from Biogen and Roche. CT, ALBM have received personal fees and non-financial support from Roche and Biogen. All these competing interest are outside the submitted work. AR, OB, LD, KG, LG, EL, CL, MM, MM, CO, GN, AP, MP, MS, CV have no competing interests.
Figures
References
-
- P. Burr et A. K. R. Reddivari, « Spinal Muscle Atrophy », in StatPearls, Treasure Island (FL): StatPearls Publishing, 2023. Consulté le: 29 août 2023. [En ligne]. Disponible sur: http://www.ncbi.nlm.nih.gov/books/NBK560687/ - PubMed
-
- Mercuri E, et al. Risdiplam in types 2 and 3 spinal muscular atrophy: a randomised, placebo-controlled, dose-finding trial followed by 24 months of treatment. Euro J of Neurology. 2023;30(7):1945–56. 10.1111/ene.15499. - PubMed
-
- Finkel RS, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723–32. 10.1056/NEJMoa1702752. - PubMed
-
- Baranello G, et al. Risdiplam in type 1 spinal muscular atrophy. N Engl J Med. 2021;384(10):915–23. 10.1056/NEJMoa2009965. - PubMed
-
- Mercuri E, et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2022;21(1):42–52. 10.1016/S1474-4422(21)00367-7. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical