Neonates exposed to HIV but uninfected exhibit an altered gut microbiota and inflammation associated with impaired breast milk antibody function
- PMID: 39707483
- PMCID: PMC11662858
- DOI: 10.1186/s40168-024-01973-z
Neonates exposed to HIV but uninfected exhibit an altered gut microbiota and inflammation associated with impaired breast milk antibody function
Abstract
Background: Infants exposed to HIV but uninfected have altered immune profiles which include heightened systemic inflammation. The mechanism(s) underlying this phenomenon is unknown. Here, we investigated differences in neonatal gut bacterial and viral microbiome and associations with inflammatory biomarkers in plasma. Further, we tested whether HIV exposure impacts antibody-microbiota binding in neonatal gut and whether antibodies in breast milk impact the growth of commensal bacteria.
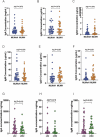
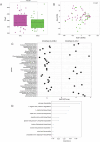
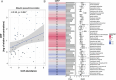
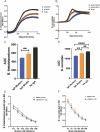
Results: Neonates exposed to HIV but uninfected (nHEU) exhibited altered gut bacteriome and virome compared to unexposed neonates (nHU). In addition, HIV exposure differentially impacted IgA-microbiota binding in neonates. The relative abundance of Blautia spp. in the whole stool or IgA-bound microbiota was positively associated with plasma concentrations of C-reactive protein. Finally, IgA from the breast milk of mothers living with HIV displayed a significantly lower ability to inhibit the growth of Blautia coccoides which was associated with inflammation in nHEU.
Conclusion: nHEU exhibits profound alterations in gut bacterial microbiota with a mild impact on the enteric DNA virome. Elevated inflammation in nHEU could be due to a lower capacity of breast milk IgA from mothers living with HIV to limit growth the of gut bacteria associated with inflammation. Video Abstract.
Keywords: Gut microbiota; Inflammation; Neonates exposed to HIV but uninfected.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Infants were recruited as part of an ongoing observational study of mother-infant pairs in Cape Town, South Africa [22]. The study was approved by the University of Cape Town’s Human Research Ethics Committee (reference 285/2012). Consent for publication: This declaration is not applicable. Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- UNAIDS. Fact Sheet-World AIDS Day 2017. Geneva: UN Joint Programme on HIV/AIDS (UNAIDS); Programme on HIV/AIDS. 2017. 978–92–9173–945–5.
-
- Slogrove AL, Cotton MF, Esser MM. Severe infections in HIV-exposed uninfected infants: clinical evidence of immunodeficiency. J Trop Pediatr. 2010;56:75–81. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

