Selective retinoid X receptor agonism promotes functional recovery and myelin repair in experimental autoimmune encephalomyelitis
- PMID: 39707547
- PMCID: PMC11662761
- DOI: 10.1186/s40478-024-01904-x
Selective retinoid X receptor agonism promotes functional recovery and myelin repair in experimental autoimmune encephalomyelitis
Abstract
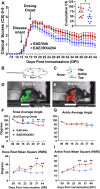
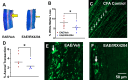
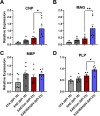
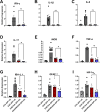
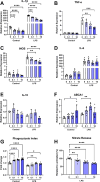
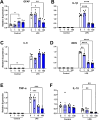
Evidence that myelin repair is crucial for functional recovery in multiple sclerosis (MS) led to the identification of bexarotene (BXT). This clinically promising remyelinating agent activates multiple nuclear hormone receptor subtypes implicated in myelin repair. However, BXT produces unacceptable hyperlipidemia. In contrast, IRX4204 selectively activates the retinoid X receptor (RXR). Given compelling links between RXR activation and increased myelin repair, we employed IRX4204 to investigate the impact of RXR agonism alone on functional recovery in mice subjected to experimental autoimmune encephalomyelitis (EAE). Since gait deficits are common in MS, we used machine learning to obtain highly sensitive and reliable measurements of sagittal hindleg joint movements for mice walking on a treadmill. IRX4204 not only blocked the progressive loss of knee and ankle movements but also reversed joint movement impairments in EAE mice. Our biochemical, transcriptional and histological measurements in spinal cord suggest these gait improvements reflect increased axon survival and remyelination and reduced inflammation. Using microglia, astrocytes and oligodendrocyte progenitor cells, we present additional data suggesting that IRX4204 may act on multiple glial subtypes to orchestrate myelin repair. These results inform the discovery of restorative neural therapeutics for MS by demonstrating that selective RXR agonism is sufficient for effective myelin repair. Moreover, our findings support the therapeutic potential of IRX4204 to promote functional recovery in MS.
Keywords: Multiple sclerosis; Neural repair; Neuroprotection; Oligodendrocyte progenitor cell; Rehabilitation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: Drs. Vuligonda and Sanders are equity holders, officers, and members of the Board of Directors of Io Therapeutics, Inc., which is developing IRX4204 for commercialization as a treatment for multiple sclerosis and other neurodegenerative diseases.
Figures


References
-
- (2016) 2016 Emerging Science Program Schedule. Neurology 87: e20–e25 Doi 10.1212/WNL.0000000000002784
-
- Akay T, Acharya HJ, Fouad K, Pearson KG (2006) Behavioral and electromyographic characterization of mice lacking EphA4 receptors. J Neurophysiol 96:642–651. 10.1152/jn.00174.2006 - PubMed
-
- Armstrong RC (1998) Isolation and characterization of immature oligodendrocyte lineage cells. Methods 16:282–292. 10.1006/meth.1998.0685 - PubMed
-
- Benedetti MG, Piperno R, Simoncini L, Bonato P, Tonini A, Giannini S (1999) Gait abnormalities in minimally impaired multiple sclerosis patients. Mult Scler 5:363–368. 10.1177/135245859900500510 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

