Small extracellular vesicles derived from umbilical cord mesenchymal stem cells alleviate radiation-induced cardiac organoid injury
- PMID: 39707562
- PMCID: PMC11662859
- DOI: 10.1186/s13287-024-04115-2
Small extracellular vesicles derived from umbilical cord mesenchymal stem cells alleviate radiation-induced cardiac organoid injury
Abstract
Background: Radiation-induced heart disease (RIHD) is one of the most serious complications of radiation therapy (RT) for thoracic tumors, and new interventions are needed for its prevention and treatment. Small extracellular vesicles (sEVs) from stem cells have attracted much attention due to their ability to repair injury. However, the role of umbilical cord mesenchymal stem cell (UCMSC)-derived sEVs in protecting cardiac organoids from radiation-induced injury and the underlying mechanisms are largely unknown.
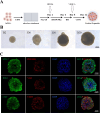
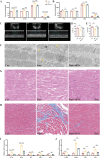
Methods: A radiation-induced cardiac organoid injury model was established by using X-ray radiation, and the optimal radiation dose of 20 Gy was determined by live/dead staining. After radiation, the cardiac organoids were treated with sEVs derived from UCMSCs, and energy metabolism, calcium transient changes and the ultrastructure of the organoids were assessed through Seahorse analysis, optical mapping and transmission electron microscopy, respectively. Confocal microscopy was used to observe the changes in mitochondrial ROS and mitochondrial membrane potential (ΔΨm). Furthermore, real-time quantitative PCR was used to verify the RNA-seq results.
Results: After X-ray radiation, the mortality of cardiac organoids significantly increased, energy metabolism decreased, and calcium transients changed. We also observed that the mitochondrial structure of cardiac organoids was disrupted and that ΔΨm was decreased. These effects could be inhibited by sEVs treatment. sEVs may protect against radiation-induced cardiac organoid injury by regulating oxidative phosphorylation and the p53 signaling pathway.
Conclusion: sEVs derived from UCMSCs can be used as a potential therapeutic strategy for radiation-induced heart disease.
Keywords: Cardiac organoids; Mesenchymal stem cells; Mitochondrial function; Radiation-induced heart disease; Small extracellular vesicles.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The iPSCs used in our research was obtained from CORIELL Institute (GM25256, USA), which appropriate consent was obtained at the time of biospecimen collection. Clinical-grade UCMSCs was obtained from China Medical Management Consulting (Beijing) LTD. CO. Human umbilical cords were provided by women who gave birth at Yantai Laiyang Central Hospital (Yantai, China). The collection of umbilical cords and the development of mesenchymal stem cells were reviewed and approved by Laiyang Central Hospital of Yantai City on November 14, 2023, with approval number of 2023-002-001. Title of the approved project was “Umbilical cord collection and development of mesenchymal stem cells”. Written informed consent was obtained from the donors participating in the study. All procedures that involved human samples were approved by the ethics committee of Academy of Military Medical Sciences (AF/SC-08/02.425). Title of the approved project was “Experimental study on intervention effect of mesenchymal stem cells derived small extracellular vesicles on radiation-induced cardiac organoid injury”. All procedures that involved animals were approved by the Institutional Animal Care and Use Committee of the Laboratory Animal Center of Academy of Military Medical Sciences (IACUC-DWZX-2023-532). Title of the approved project was “Study on protective effect of stem cells derived small extracellular vesicles on radiation-induced heart damage in mice”. Consent for publication: Not applicable. Competing interests: The authors declare no competing financial interest.
Figures






References
-
- Lee PJ, Mallik R. Cardiovascular effects of radiation therapy: practical approach to radiation therapy-induced heart disease. Cardiol Rev. 2005;13(2):80–6. 10.1097/01.crd.0000131188.41589.c5. - PubMed
-
- Donnellan E, Phelan D, McCarthy CP, et al. Radiation-induced heart disease: a practical guide to diagnosis and management. Cleve Clin J Med. 2016;83(12):914–22. 10.3949/ccjm.83a.15104. - PubMed
-
- Boero IJ, Paravati AJ, Triplett DP, et al. Modern Radiation Therapy and Cardiac outcomes in breast Cancer. Int J Radiat Oncol Biol Phys. 2016;94(4):700–8. 10.1016/j.ijrobp.2015.12.018. Epub 2015 Dec 17. - PubMed
-
- Boekel NB, Schaapveld M, Gietema JA, et al. Cardiovascular Disease Risk in a large, Population-based cohort of breast Cancer survivors. Int J Radiat Oncol Biol Phys. 2016;94(5):1061–72. Epub 2015 Dec 14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

