Epigenetic Regulation by Histone Methylation and Demethylation in Freeze-Tolerant Frog Kidney
- PMID: 39707620
- PMCID: PMC11662150
- DOI: 10.1002/cbf.70036
Epigenetic Regulation by Histone Methylation and Demethylation in Freeze-Tolerant Frog Kidney
Abstract
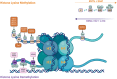
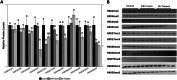
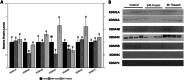
The wood frog (Rana sylvatica) endures whole-body freezing over the winter, with extensive extracellular ice formation and halted physiological activities. Epigenetic mechanisms, including reversible histone lysine methylation, enable quick alterations in gene expression, helping to maintain viability during freeze-thaw cycles. The present study evaluated eight histone lysine methyltransferases (KMTs), 10 histone lysine demethylases (KDMs), and 11 histone marks in wood frog kidneys. Using immunoblotting, significant changes in relative protein levels of multiple KMTs and KDMs were observed in response to freezing, with variable alterations during thawing. Specifically, the repressive methyl marks H3K27me1 and H4K20me3 significantly decreased during freezing, whereas H3K9me3, H3K27me3, and H3K36me2 decreased during thawing. These results demonstrate that the regulation of histone methylation and demethylation play crucial roles in controlling gene expression over the freeze-thaw cycle and the maintenance of normal renal physiology.
Keywords: Rana sylvatica; freeze tolerance; gene expression; hypometabolism; kidney.
© 2024 he Author(s). Cell Biochemistry and Function published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Lee R. E., Costanzo J. P., Davidson E. C., and Layne J. R., “Dynamics of Body Water During Freezing and Thawing in a Freeze‐Tolerant Frog (Rana sylvatica),” Journal of Thermal Biology 17, no. 4–5 (1992): 263–266, 10.1016/0306-4565(92)90064-M. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

