De-escalating and discontinuing disease-modifying therapies in multiple sclerosis
- PMID: 39707906
- PMCID: PMC12073975
- DOI: 10.1093/brain/awae409
De-escalating and discontinuing disease-modifying therapies in multiple sclerosis
Abstract
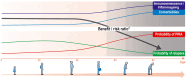
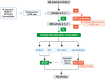
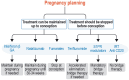
The development of disease-modifying therapies (DMTs) for the treatment of multiple sclerosis (MS) has been highly successful in recent decades. It is now widely accepted that early initiation of DMTs after disease onset is associated with a better long-term prognosis. However, the question of when and how to de-escalate or discontinue DMTs remains open and critical. This topic was discussed during an international focused workshop organized by the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) in 2023. The aim was to review the current evidence on the rationale for, and the potential pitfalls of, treatment de-escalation in MS. Several clinical scenarios emerged, mainly driven by a change in the benefit-risk ratio of DMTs over the course of the disease and with ageing. The workshop also addressed the issue of de-escalation by the type of DMT used and in specific situations, including pregnancy and paediatric onset MS. Finally, we provide practical guidelines for selecting appropriate patients, defining de-escalation and monitoring modalities and outlining unmet needs in this field.
Keywords: ageing; de-escalation; discontinuation; disease-modifying therapy; multiple sclerosis; pregnancy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
G.A. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Jannsen, Merck, Novartis, Roche and Sanofi-Genzyme. J.D.L. has received honoraria for acting as a member of Scientific Advisory Boards for Abbvie, Alexion, Argenx, Moderna, Sanofi and Takeda as well as speaker honoraria and travel support from Alexion, Argenx, Biogen, Roche Pharma AG, Merck, Moderna, Novartis, and Sanofi. His research is funded by the European Union, Deutsche Forschungsgesellschaft (DFG), Alexion, Argenx, Moderna, and Roche Pharma AG. E.M. reports research support from Biogen and ARSEP foundation and personal fees for lectures and advisory boards from Biogen, Janssen, Merck, Novartis, Roche, Sanofi, Teva. M.P.A. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Jannsen, Merck, Novartis, Roche, Sanofi-Genzyme, Sandoz and Celgene BMS, and research grants by Novartis, Merck and Roche. G.B. has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Janssen, Lilly, Merck, Novartis, Roche, Sanofi-Genzyme and Teva, and received honoraria for consulting Biogen, Celgene/BMS, Janssen, Merck, Novartis, Roche, Sanofi-Genzyme and Teva. He has received unrestricted research grants from Celgene/BMS and Novartis. H.B. received institutional (Monash University) funding from Biogen, Hoffmann-La Roche Ltd, Merck, UCB, TEVA and Novartis for research projects, speaker honoraria and steering committee activities. He has received travel support from Novartis and Merck. O.C.: NIHR Research Professor (RP-2017-08-ST2-004); over the last 2 years, member of independent DSMB for Novartis; she gave a teaching talk in a Merck local symposium, and contributed to an Advisory Board for Biogen; she is Deputy Editor of Neurology, for which she receives an honorarium; she has received research grant support from the MS Society of Great Britain and Northern Ireland, the NIHR UCLH Biomedical Research Centre, the Rosetree Trust, the National MS Society, and the NIHR-HTA. A.C.C. has received grant from Instituto de Salud Carlos III, Spain; JR19/00007. T.D. received speaker fees, research support, travel support, and/or served on Advisory Boards or Steering Committees of Alexion, Novartis, Merck, Biogen, GeNeuro, MedDay, Roche, and Sanofi Genzyme. F.D.P. has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations) or travel funding from Amgen, Bayer, Biogen, Celgene, Horizon, Merck, Novartis, Sanofi-Genzyme, Roche and Teva. G.E. is the technical and scientific coordinator (RST) of the RHU Primus since 2021, having a partnership with the industrials Merck, Biogen, Pixyl for this research and economic program. C.E. has received personal compensation for consulting or speaking from Biogen-Idec, BMS, EMD-Serono, Novartis, Roche, and Sanofi-Genzyme. R.G. received support for scientific meetings and courses from Bayer, Biogen, Merck, Novartis, Jasen and honoraria for advisory work or talks from Biogen, Novartis, UCB, MIAC. C.G.: The University Hospital Basel (USB) and the Research Center for Clinical neuroimmunology and Neuroscience (RC2NB), as the employers of Cristina Granziera, have received the following fees which were used exclusively for (research support from Siemens, GeNeuro, Genzyme-Sanofi, Biogen, Roche. They also have received advisory board and consultancy fees from Actelion, Genzyme-Sanofi, Novartis, GeNeuro, Merck, Biogen and Roche; as well as speaker fees from Genzyme-Sanofi, Novartis, GeNeuro, Merck, Biogen and Roche. H.P.H. received honoraria for serving on SC, DMC or speaking at symposia from Biogen, BMS Celgene, Merck, Novartis, Roche, Sanofi, TG Therapeutics; for work as section chief editor of Frontiers Neurology/Immunology. M.I. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Alexion, Biogen, Brystol Meyer Squibb, Janssen, Merck, Novartis, Roche and Sanofi-Genzyme. L.K.: Ludwig Kappos’ institutions (University Hospital Basel and RC2NB) have received compensation that was used exclusively to support research, for the following: consultancy fees from Bayer HealthCare, Biogen, Bristol Myers Squibb, Celltrion Inc., Eli Lilly SA, EMD Serono Research and Development, GlaxoSmithKline, Galapagos NV, Janssen, Japan Tobacco Inc., Kiniksa Pharmaceuticals, Merck Healthcare AG, Minoryx, Neurostatus UHB AG, Novartis, Roche, Sanofi, Santhera Pharmaceuticals, Shionogi BV, Wellmera AG, and Zai Lab; contracted research from the European Union, InnoSwiss, Merck Healthcare AG, Novartis, Neurostatus UHB AG, Sanofi, and Roche; speaker fees and support of educational activities from Bristol Myers Squibb, Janssen, Roche, Sanofi, Merck, and Novartis; serving on the steering committee or advisory board for Biogen, EMD Serono Research and Development, Genentech, Janssen, Novartis, Clene Nanomedicine Inc., and Sanofi. H.K. has received personal compensation for consulting, serving on a scientific advisory board, speaking, or participated in clinical trials from Merck, Sanofi, Novartis, Roche, Celgene, Jansen Cilag, Biogen, Sandoz, Argenx and Alexion. Research funding from Academy of Finland Strategic Research Council (31213358415) and the State funding for university-level health research, Tampere University Hospital, Finland (9AC042). A.L.G. has received grant support and awards from the Patient-Centered Outcomes Research Institute, the National MS Society, and Atara Biotherapeutics. She currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review (ICER). M.M. served on scientific advisory board, as consultant for, received support for congress participation or speaker honoraria from Biogen, Sanofi, Roche, Novartis, Merck, Alexion and Bristol Myers Squibb. The Danish MS Registry received research support from Biogen, Genzyme, Roche, Merck and Novartis. R.M. serves on scientific advisory boards for Amgen/Horizon Therapeutics, UCB and Roche; and has received funding for travel and fees from Amgen, Alexion, Biogen, Roche. X.M. has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Abbvie, Actelion, Alexion, Biogen, Bristol-Myers Squibb/Celgene, EMD Serono, Genzyme, Hoffmann-La Roche, Immunic Therapeutics, Janssen Pharmaceuticals, Medday, Merck, Mylan, Nervgen, Novartis, Sandoz, Sanofi-Genzyme, Teva Pharmaceutical, TG Therapeutics, Excemed, MSIF and NMSS. M.P.M. has received speaking fees from Sanofi, Biogen, Merck, Serono, Novartis, Roche, Boehringer Ingelheim, Bristol Myers Squibb and Teva. B.N. has received research funding from the US National Multiple Sclerosis Society, the US National Institutes of Health, the US Department of Defense, PCORI, Genentech, and Axsome Therapeutics. He has received consulting honoraria from TG Therapeutics and Alkermes. J.O. has received grant funding from Brain Canada, MS Canada, the National MS Society, Biogen-Idec, Roche, and EMD-Serono and has received personal compensation for consulting or speaking from: Biogen-Idec, BMS, EMD-Serono, Eli-Lilly, Horizon Therapeutics, Novartis, Roche, and Sanofi-Genzyme. C.O.G. has received speaking and/or consultancy fees from Alexion, Biogen, BMS, Jannsen, Merck, Novartis, Roche, Sanofi, and Teva. F.P. has received research grants from Janssen, Merck KGaA and UCB, and fees for serving on DMC in clinical trials with Chugai, Lundbeck and Roche, and preparation of expert witness statement for Novartis. L.P. has received personal fees and non-financial support from Biogen, Bristol-Myers Squibb, Merck, Novartis, Roche, Sanofi and Viatris. J.S.G. received compensation in the last 24 months for consulting services and speaking honoraria from BMS, Sanofi, Merck, Janssen, Novartis, and Roche, is scientific director of Revista de Neurología and member of the editorial committee of Multiple Sclerosis Journal, for which he receives an honorarium. He has received research support from Fondo de Investigación en Salud (PI19/00950 and PI22/00750) from Instituto de Salud Carlos III, Spain. F.S. has served on scientific advisory boards for, served as consultant for, received support for congress participation or received speaker honoraria from Biogen, Bristol Myers Squibb, Lundbeck, Merck, Novartis, Roche and Sanofi Genzyme, and has received research support from Biogen, Merck, Novartis, Roche and Sanofi Genzyme for his laboratory. K.S. has received personal compensation for consulting from Biogen, Celgene, GeNeuro, Merck, Novartis, Polpharma, Sanofi, Roche, TG Therapeutics, and received research support from Merck and Roche. A.S. has received research grants from The Turkish Multiple Sclerosis Society; and research grants from The Scientific and Technological Research Council Of Turkey & Istanbul University-Cerrahpasa Research Support Funds and has received honoraria or consultancy fees for participating to advisory boards, giving educational lectures and/or travel and registration coverage for attending scientific congresses or symposia from F. Hoffmann-La Roche Ltd, Merck-Serono, Novartis, Teva, Biogen Idec/Gen Pharma of Turkey, Alexion, Abdi Ibrahim Ilaç and Ali Raif Ilaç. E.T. has received honorarium for consulting work from Biogen, Janssen, Merck, Novartis, and Roche in the last 5 years. She has received travel grants to attend or speak at educational meetings from Biogen, Merck, Roche, Takeda and Novartis. V.v.P. received travel grants from Merck Healthcare KGaA (Darmstadt, Germany), Biogen, Sanofi, Bristol Meyer Squibb, Almirall and Roche. His institution has received research grants and consultancy fees from Roche, Biogen, Sanofi, Merck Healthcare KGaA (Darmstadt, Germany), Bristol Meyer Squibb, Janssen, Almirall, Novartis Pharma, and Alexion. S.V. has received non-personal consulting and lecturing fees, travel grants and unconditional research support from Biogen, Janssen, Merck, Novartis, Roche, Sandoz and Sanofi. B.W.G. served as a consultant for Biogen, EMD Serono, Novartis, Genentech, Celgene/Bristol Meyers Squibb, Sanofi Genzyme, Bayer, Janssen, Labcorp, Horizon and SANA. She also has received grant/research support from Novartis, Biogen and Horizon/Amgen. She serves in the editorial board for Children, CNS Drugs, MS International, Journal of Neurology and Frontiers Epidemiology. F.Z. has recently received research grants and/or consultation funds from Biogen, German Ministry of Education and Research (BMBF), Bristol-Meyers-Squibb, Celgene, German Research Foundation (DFG), Janssen, Max-Planck-Society (MPG), Merck Serono, Novartis, Progressive MS Alliance (PMSA), Roche, Sanofi Genzyme and Sandoz. M.T. has received compensation for consulting services, speaking honoraria and research support from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Immunic Therapeutics, Janssen, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Viela Bio and Teva Pharmaceuticals. Data Safety Monitoring Board for Parexel and UCB Biopharma, Relapse Adjudication Committee for IMCYSE SA. E.I. has received honoraria for serving on advisory boards for Biogen, Sanofi-Genzyme, and Merck and speaker's fees from Biogen and Sanofi-Genzyme. She has received an unrestricted research grant from Sanofi-Genzyme and research funding from Region Stockholm Clinical Research Appointment, Neuro Sweden and the Lindholm Fredholms Foundation. B.S. has received lecturing fees from Biogen Idec, Merck-Serono, Novartis, Sanofi and Janssen, and unconditional research support (to the institution) from Merck-Serono, Novartis, and Roche. The other authors report no competing interests.
Figures

References
-
- Tintore M, Vidal-Jordana A, Sastre-Garriga J. Treatment of multiple sclerosis—Success from bench to bedside. Nat Rev Neurol. 2019;15:53–58. - PubMed
-
- Lünemann JD, Ruck T, Muraro PA, Bar-Or A, Wiendl H. Immune reconstitution therapies: Concepts for durable remission in multiple sclerosis. Nat Rev Neurol. 2020;16:56–62. - PubMed
-
- Chalmer TA, Baggesen LM, Nørgaard M, et al. . Early versus later treatment start in multiple sclerosis: A register-based cohort study. Eur J Neurol. 2018;25:1262-e110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

