Rare dysfunctional SCN2A variants are associated with malformation of cortical development
- PMID: 39707911
- PMCID: PMC11908663
- DOI: 10.1111/epi.18234
Rare dysfunctional SCN2A variants are associated with malformation of cortical development
Abstract
Objective: SCN2A encodes the voltage-gated sodium (Na+) channel α subunit NaV1.2, which is important for the generation and forward and back propagation of action potentials in neurons. Genetic variants in SCN2A are associated with a spectrum of neurodevelopmental disorders. However, the mechanisms whereby variation in SCN2A leads to disease remains incompletely understood, and the full spectrum of SCN2A-related disorders may not be fully delineated.
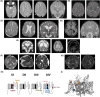
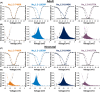
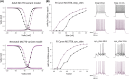
Methods: Here, we identified seven de novo heterozygous variants in SCN2A in eight individuals with developmental and epileptic encephalopathy (DEE) accompanied by prominent malformation of cortical development (MCD). We characterized the electrophysiological properties of Na + currents in human embryonic kidney (HEK) cells transfected with the adult (A) or neonatal (N) isoform of wild-type (WT) and variant NaV1.2 using manual and automated whole-cell voltage clamp recording.
Results: The neonatal isoforms of all SCN2A variants studied exhibit gain of function (GoF) with a large depolarized shift in steady-state inactivation, creating a markedly enhanced window current common across all four variants tested. Computational modeling demonstrated that expression of the NaV1.2-p.Met1770Leu-N variant in a developing neocortical pyramidal neuron results in hyperexcitability.
Significance: These results support expansion of the clinical spectrum of SCN2A-related disorders and the association of genetic variation in SCN2A with MCD, which suggests previously undescribed roles for SCN2A in fetal brain development.
Keywords: SCN2A; Nav1.2; developmental and epileptic encephalopathy; malformation of cortical development; voltage‐gated sodium channel.
© 2024 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
None of the authors has any conflict of interest to disclose.
Figures


References
-
- Wolff M, Brunklaus A, Zuberi SM. Phenotypic spectrum and genetics of SCN2A‐related disorders, treatment options, and outcomes in epilepsy and beyond. Epilepsia. 2019;60(Suppl 3):S59–S67. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

