Prognostic factors and impact of management strategies for status epilepticus: The STEPPER study in the Emilia-Romagna region, Italy
- PMID: 39707958
- PMCID: PMC11908670
- DOI: 10.1111/epi.18227
Prognostic factors and impact of management strategies for status epilepticus: The STEPPER study in the Emilia-Romagna region, Italy
Abstract
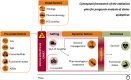
Objective: The STEPPER (Status Epilepticus in Emilia-Romagna) study aimed to investigate the clinical characteristics, prognostic factors, and treatment approaches of status epilepticus (SE) in adults of the Emilia-Romagna region (ERR), Northern Italy.
Methods: STEPPER, an observational, prospective, multicentric cohort study, was conducted across neurology units, emergency departments, and intensive care units of the ERR over 24 months (October 2019-October 2021), encompassing incident cases of SE. Patients were followed up for 30 days.
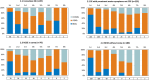
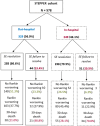
Results: A total of 578 cases were recruited (56% female, mean age = 70 years, 32% with previous diagnosis of epilepsy, 43% with in-hospital onset, 35% stuporous/comatose, 46% with nonconvulsive SE). Etiology was known in 87% (acute 43%, remote 24%, progressive 17%, definite epileptic syndrome 3%). The mean pre-SE Rankin Scale score was 2, the Status Epilepticus Severity Score was ≥4 in 33%, the Epidemiology-Based Mortality Score in Status Epilepticus score was ≥64 in 61%, and 34% were refractory. The sequence of treatments followed current clinical practice guidelines in 63%. Benzodiazepines (BDZs) were underused as first-line therapy (71%), especially in in-hospital onset cases; 15% were treated with continuous intravenous anesthetic drugs. Mortality was 24%; 63% of survivors had functional worsening. At the two-step multivariable analysis, incorrect versus correct treatment sequence with correct BDZ dose was the strongest predictor of failure to resolve SE in the in-hospital group (odds ratio [OR] = 4.42, 95% confidence interval [CI] = 1.86-10.5), with a similar trend in the out-of-hospital group (OR = 2.22, 95% CI = .98-5.02). In turn, failure to resolve was the strongest predictor of 30-day mortality (OR = 11.3, 95% CI = 4.16-30.9, out-of-hospital SE; OR = 6.42, 95% CI = 2.79-14.8, in-hospital SE) and functional worsening (OR = 5.83, 95% CI = 2.05-16.6, out-of-hospital SE; OR = 9.30, 95% CI 2.22-32.3, in-hospital SE).
Significance: The STEPPER study offers insights into real-world SE management, highlighting its significant morbidity and functional decline implications. Although nonmodifiable clinical factors contribute to SE severity, modifiable factors such as optimized first-line therapies and adherence to guidelines can potentially influence prognosis.
Keywords: EEG; antiseizure medications; cohort studies; natural history studies; status epilepticus.
© 2024 The Author(s). Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
F.B. has received grants or contracts from the Italian Ministry of Health; has received consulting fees from Eisai, Angelini, UCB, and Takeda; has received support for attending meetings and/or travel from LivaNova; and has been a member of the Lafora advocacy group executive board. E.C. has received support for attending meetings and/or travel from Eisai and Angelini Pharma. L.D.V. has received grants or contracts from the Italian Ministry of Health and has received support for attending meetings and/or travel from Angelini. I.F. has received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Angelini; and has received support for attending meetings and/or travel from Angelini. S.M. has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from UCB Pharma, Eisai, Jazz Pharmaceuticals, and Angelini; and has received support for attending meetings and/or travel from UCB Pharma, Eisai, Angelini, and Jazz Pharmaceuticals. R.M. has received support for attending meetings and/or travel from Angelini and Eisai. N.O. has received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Eisai. M.R. has participated on the steering committee of the FibER trial. A.Z. has received consulting fees from Boehringer‐Ingelheim, CSL Behring, and Angels Initiative; has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events AstraZeneca, Daiichi Sankyo, and Angels Initiative; has received payment for expert testimony (Alexion–AstraZeneca); and has participated on a data safety monitoring or advisory board for Bayer and AstraZeneca. L.V. has received grants or contracts from the Italian Ministry of Health. None of the other authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
References
-
- Lattanzi S, Giovannini G, Brigo F, Orlandi N, Trinka E, Meletti S. Acute symptomatic status epilepticus: splitting or lumping? A proposal of classification based on real‐world data. Epilepsia. 2023;64:e200–e206. - PubMed
-
- Lattanzi S, Trinka E, Brigo F, Meletti S. Clinical scores and clusters for prediction of outcomes in status epilepticus. Epilepsy Behav. 2023;140:109110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

