A Bioequivalence Study of Two Formulations of Oral Semaglutide in Healthy Participants
- PMID: 39708086
- PMCID: PMC11794934
- DOI: 10.1007/s13300-024-01674-8
A Bioequivalence Study of Two Formulations of Oral Semaglutide in Healthy Participants
Abstract
Introduction: The glucagon-like peptide-1 (GLP-1) analogue semaglutide is approved as an oral formulation for the treatment of type 2 diabetes. This study aimed to confirm bioequivalence between a new, second-generation (2G) oral semaglutide formulation (1.5, 4 and 9 mg) and the initially approved first-generation (1G) formulation (3, 7 and 14 mg).
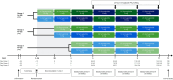
Methods: This was a randomised, multicentre, open-label, full replicate crossover study to confirm bioequivalence between 2G and 1G oral semaglutide formulations at steady-state (SS) in healthy participants (NCT05227196). Participants were recruited to three groups. In each group, participants were randomised to one of two alternating sequences comparing once-daily oral semaglutide treatment of 9 and 14 mg (group 1), 4 and 7 mg (group 2) or 1.5 and 3 mg (group 3) at SS. Treatment duration was 20 weeks, comprising four 5-week steady-state periods on alternating formulations. Repeated 24-h blood sampling at the end of each steady-state period supported pharmacokinetic analysis. Co-primary endpoints were area under the semaglutide plasma concentration-time curve during a dosing interval at SS (AUC0-24h,SS) and maximum semaglutide plasma concentration at SS (Cmax, 0-24h,SS). Bioequivalence for co-primary endpoints was assessed using European Medicines Agency (EMA), U.S. Food and Drug Administration (FDA) and Japan Pharmaceuticals and Medical Devices Agency (PMDA) criteria. Safety was monitored.
Results: A total of 222, 201 and 123 participants were recruited into groups 1, 2 and 3, respectively. The prespecified EMA, FDA and PMDA bioequivalence criteria were met for 2G versus 1G oral semaglutide for all three dose levels (1.5 vs 3 mg, 4 vs 7 mg and 9 vs 14 mg). The safety profile of 2G oral semaglutide was consistent with 1G oral semaglutide.
Conclusions: The 2G oral semaglutide formulation was confirmed as bioequivalent to 1G oral semaglutide, with no new safety concerns identified.
Trial registration: ClinicalTrials.gov identifier, NCT05227196.
Keywords: Antidiabetic drug; Bioavailability; Bioequivalence; GLP-1 analogue; Glycaemic control; Incretin therapy; Semaglutide; Type 2 diabetes.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Mette Søndergaard Nielsen—Employee and shareholder of Novo Nordisk A/S. Lise Brøndsted—Employee and shareholder of Novo Nordisk A/S. Martin Kankam—One of the clinical investigators who participated in the conduct of the study, which was sponsored by Novo Nordisk; employee at Altasciences and has received clinical trial funding from Amgen, Biogen, Biohaven, Camino, DynPort, Eli Lilly, EncuraGen, NIH/DMID, Staidson and Vertex. Gaetano Morelli—Employee at Altasciences. David Nguyen—Employee at Altasciences. Trine Vang Skjøth—Employee and shareholder of Novo Nordisk A/S. Usha Rani Patted—Employee of Novo Nordisk India Private Ltd. Marloes van Hout—Employee and shareholder of Novo Nordisk A/S. Ethical approval: The study protocol was approved by appropriate health authorities according to local guidelines and by the Institutional Review Board/Independent Ethics Committee Institutional Review Board, listed in the Supplementary Appendix. The study was conducted in accordance with the Declaration of Helsinki and International Council for Harmonization (ICH) Good Clinical Practice guidelines. Participants provided written informed consent prior to commencement of any study-related activities.
Figures


References
-
- Tran S, Retnakaran R, Zinman B, Kramer CK. Efficacy of glucagon-like peptide-1 receptor agonists compared to dipeptidyl peptidase-4 inhibitors for the management of type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2018;20(Suppl 1):68–76. - PubMed
-
- Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

