Melatonin mitigates intervertebral disc degeneration by suppressing NLRP3 inflammasome activation via the EGR1/DDX3X pathway
- PMID: 39708233
- PMCID: PMC11670809
- DOI: 10.1096/fj.202302453RRR
Melatonin mitigates intervertebral disc degeneration by suppressing NLRP3 inflammasome activation via the EGR1/DDX3X pathway
Abstract
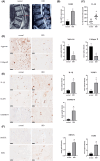
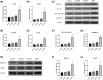
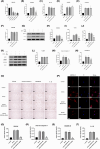
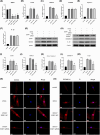
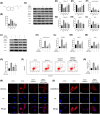
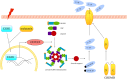
Intervertebral disc degeneration (IVDD), is one of the leading causes of low back pain. Inflammation is considered to be the main pathophysiological process of IVDD. The nucleotide-binding domain and leucine-rich pyrin domain containing 3 (NLRP3) inflammasome-mediated inflammatory responses are critically involved in the progression of IVDD. Melatonin is known for its anti-inflammatory and antioxidant effects. However, little is known about the potential effects of melatonin in the pathological process of IVDD. We found that the expression of EGR1, DDX3X, and NLRP3 inflammasome increased and extracellular matrix (ECM) degraded in IVDD. With the application of EGR1 siRNA, the expression of DDX3X and the activation of NLRP3 inflammasome were inhibited in stress-induced NP cells. DDX3X/NLRP3 was regulated on dependence of EGR1. Besides, the utility of melatonin mitigated the EGR1-induced overproduction of DDX3X and activation of NLRP3 inflammasome, thus protecting cells from pyroptosis and ECM degradation. In vivo, in a rat IVDD model, melatonin was found to be able to delay the development of IVDD by imageological and histological evaluation. In conclusion, our study demonstrated that melatonin prevented IVDD progression by regulating EGR1/DDX3X/NLRP3 axis. Our study provides insight into melatonin as a new target for therapeutic approaches for IVDD.
Keywords: DDX3X; EGR1; NLRP3 inflammasome; intervertebral disc degeneration; melatonin; nucleus pulposus.
© 2024 Federation of American Societies for Experimental Biology.
Figures

References
-
- Yang S, Zhang F, Ma J, Ding W. Intervertebral disc ageing and degeneration: the antiapoptotic effect of oestrogen. Ageing Res Rev. 2020;57:100978. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

