An Expert Consensus Statement on Biomarkers of Aging for Use in Intervention Studies
- PMID: 39708300
- PMCID: PMC11979094
- DOI: 10.1093/gerona/glae297
An Expert Consensus Statement on Biomarkers of Aging for Use in Intervention Studies
Abstract
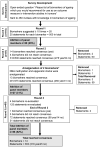
Biomarkers of aging serve as important outcome measures in longevity-promoting interventions. However, there is limited consensus on which specific biomarkers are most appropriate for human intervention studies. This work aimed to address this need by establishing an expert consensus on biomarkers of aging for use in intervention studies via the Delphi method. A 3-round Delphi study was conducted using an online platform. In Round 1, expert panel members provided suggestions for candidate biomarkers of aging. In Rounds 2 and 3, they voted on 500 initial statements (yes/no) relating to 20 biomarkers of aging. Panel members could abstain from voting on biomarkers outside their expertise. Consensus was reached when there was ≥70% agreement on a statement/biomarker. Of the 460 international panel members invited to participate, 116 completed Round 1, 87 completed Round 2, and 60 completed Round 3. Across the 3 rounds, 14 biomarkers met consensus that spanned physiological (eg, insulin-like growth factor 1, growth-differentiating factor-15), inflammatory (eg, high sensitivity C-reactive protein, interleukin-6), functional (eg, muscle mass, muscle strength, hand grip strength, Timed-Up-and-Go, gait speed, standing balance test, frailty index, cognitive health, blood pressure), and epigenetic (eg, DNA methylation/epigenetic clocks) domains. Expert consensus identified 14 potential biomarkers of aging which may be used as outcome measures in intervention studies. Future aging research should identify which combination of these biomarkers has the greatest utility.
Keywords: Consensus; Delphi method; Longevity.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Gerontological Society of America.
Conflict of interest statement
J.A.B reports receiving research funding and materials from Pfizer, Calico, Elysium Health, and Metro International Biotech and consulting fees from Pfizer, Elysium Health, Altimmune, and Cytokinetics. M.E. received research funding from the National Institutes of Health and the Alzheimer’s Association and consults with Nestle and Annovis Bio. W.J.H. is a paid consultant for Bayer Healthcare. P.K.J. is a paid consultant to Humanity Inc., a company focused on measuring and developing interventions for Biological Age. P.K.J. is partly remunerated under a Humanity Inc. share option scheme. P.K.J. is founder of Geromica, a consultancy providing advice on measurement of health and aging. D.W.L. has received funding from, and is a scientific advisory board member of Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases. F.M. has equity interest in TLL The Longevity labs and Samsara Therapeutics. A.B.M. is cofounder of Chi Longevity and Chief Medical Officer of NU. S.P. reports non-financial support from Enhanced Recovery, other from Exerkine, personal fees from Nestle Health Sciences, outside the submitted work; In addition, S.P. has a patent 3052324 issued to Exerkine, and a patent 16/182891 issued to Exerkine. D.S. has received honoraria from Pfizer, Amgen, and Abbott Nutrition. S.S. received consulting fees from Abbott Laboratories Sdn Bhd. Z.H.-S. has received speaker fees/honoraria from pharmaceutical companies including Kyowa Kirin, UCB, and Ascendis, in recent years. He is the editor of
Figures


References
-
- Prospect WP. Release Note about Major Differences in Total Population Estimates for Mid-2021 Between 2019 and 2022 Revisions. United Nations Department of Economic and Social Affairs, Population Division; 2022. https://population.un.org/wpp/Publications/Files/WPP2022_Release-Note-re...
-
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and other Tools) Resource. Food and Drug Administration (US); 2016. https://www.ncbi.nlm.nih.gov/books/NBK326791/ Co-published by National Institutes of Health (US).
-
- Moqri M, Herzog C, Poganik JR, et al. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell. 2023;186(18):3758–3775. https://doi.org/ 10.1016/j.cell.2023.08.003 - DOI - PMC - PubMed
-
- Belsky DW, Caspi A, Corcoran DL, et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. eLife. 2022;11:e73420. https://doi.org/ 10.7554/eLife.73420 - DOI - PMC - PubMed
-
- Behr LC, Simm A, Kluttig A, Grosskopf A.. 60 years of healthy aging: on definitions, biomarkers, scores and challenges. Ageing Res Rev. 2023;88:101934. https://doi.org/ 10.1016/j.arr.2023.101934 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

