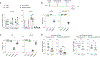Inflammation switches the chemoattractant requirements for naive lymphocyte entry into lymph nodes
- PMID: 39708807
- PMCID: PMC11845304
- DOI: 10.1016/j.cell.2024.11.031
Inflammation switches the chemoattractant requirements for naive lymphocyte entry into lymph nodes
Abstract
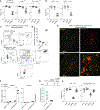
Sustained lymphocyte migration from blood into lymph nodes (LNs) is important for immune responses. The CC-chemokine receptor-7 (CCR7) ligand CCL21 is required for LN entry but is downregulated during inflammation, and it has been unclear how recruitment is maintained. Here, we show that the oxysterol biosynthetic enzyme cholesterol-25-hydroxylase (Ch25h) is upregulated in LN high endothelial venules during viral infection. Lymphocytes become dependent on oxysterols, generated through a transcellular endothelial-fibroblast metabolic pathway, and the receptor EBI2 for inflamed LN entry. Additionally, Langerhans cells are an oxysterol source. Ch25h is also expressed in inflamed peripheral endothelium, and EBI2 mediates B cell recruitment in a tumor model. Finally, we demonstrate that LN CCL19 is critical in lymphocyte recruitment during inflammation. Thus, our work explains how naive precursor trafficking is sustained in responding LNs, identifies a role for oxysterols in cell recruitment into inflamed tissues, and establishes a logic for the CCR7 two-ligand system.
Keywords: CCL19; CCL21; GPR183; cholesterol metabolites; high endothelial venules; lymph nodes; lymphocyte trafficking; stromal cells; tumors; viral infection.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures