ANXA1-FPR2 axis mitigates the susceptibility to atrial fibrillation in obesity via rescuing AMPK activity in response to lipid overload
- PMID: 39709478
- PMCID: PMC11662704
- DOI: 10.1186/s12933-024-02545-z
ANXA1-FPR2 axis mitigates the susceptibility to atrial fibrillation in obesity via rescuing AMPK activity in response to lipid overload
Abstract
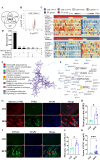
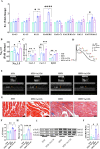
Atrial fibrillation (AF) is the most prevalent arrhythmia in clinical practice, and obesity serves as a significant risk factor for its development. The underlying mechanisms of obesity-related AF remain intricate and have yet to be fully elucidated. We have identified FPR2 as a potential hub gene involved in obesity-related AF through comprehensive analysis of four transcriptome datasets from AF patients and one transcriptome dataset from obese individuals, and its expression is up-regulated in both AF and obese individuals. Interestingly, ANXA1, the endogenous ligand of FPR2, was found to exhibit differential expression with AF and obesity. Specifically, it was observed to be down-regulated in AF patients but up-regulated in obese individuals. The susceptibility to AF in obese mice induced by high-fat diet (HFD) was increased following with the FPR2 blocker Boc-2.The administration of exogenous ANXA1 active peptide chain Ac2-26 can mitigate the susceptibility to AF in obese mice by attenuating atrial fibrosis, lipid deposition, oxidative stress injury, and myocardial cell apoptosis. However, this protective effect against AF susceptibility is reversed by AAV9-shAMPK-mediated AMPK specific knockdown in the myocardium. The vitro experiments demonstrated that silencing ANXA1 exacerbated lipid deposition, oxidative stress injury, and apoptosis induced by palmitic acid (PA) in cardiomyocytes. Additionally, Ac2-26 effectively mitigated myocardial lipid deposition, oxidative stress injury, and apoptosis induced by PA. These effects were impeded by FPR2 inhibitors Boc-2 and WRW4. The main mechanism involves the activation of AMPK by ANXA1 through FPR2 in order to enhance fatty acid oxidation in cardiomyocytes, thereby ultimately leading to a reduction in lipid accumulation and associated lipotoxicity. Our findings demonstrate that the ANXA1-FPR2 axis plays a protective role in obesity-associated AF by alleviating metabolic stress in the atria of obese mice, thereby emphasizing its potential as a promising therapeutic target for AF.
Keywords: AMP-activated protein kinase; ANXA1-FPR2; Atrial fibrillation; Lipotoxicity; Obesity.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

