Clinical characteristics of EGFR-ctDNA shedders in EGFR-mutant NSCLC patients
- PMID: 39709717
- PMCID: PMC11832947
- DOI: 10.1016/j.tranon.2024.102228
Clinical characteristics of EGFR-ctDNA shedders in EGFR-mutant NSCLC patients
Abstract
Background: Circulating tumor DNA (ctDNA) revolutionized the molecular diagnostics of lung cancer by enabling non-invasive, sensitive identification of actionable mutations. However, ctDNA analysis may be challenging due to tumor shedding variability, leading to false negative results. This study aims to understand the determinants for ctDNA shedding based on clinical characteristics of lung cancer patients, for a better interpretation of false negative results to be considered when ordering ctDNA analysis for clinical practice.
Methods: Blood samples were collected from patients with stage IV EGFR-mutated (mEGFR) NSCLC before treatment and monitored until disease progression. EGFR was assessed on tissue by standard procedures, while EGFR status on ctDNA was tested using dPCR at baseline and at the first reassessment. NGS was used to evaluate patients mutational status at the progression of the disease.
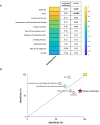
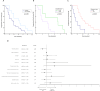
Results: A total of 40 mEGFR tissue samples were collected. Plasma samples were analyzed for mEGFR before starting the first line, 65 % of patients had detectable mEGFR in ctDNA ("shedders"). Higher ECOG PS (p = 0.04), bilateral localization of primary tumor (p = 0.04), and the presence of intrathoracic/extrathoracic disease (p = 0.05), were associated to mEGFR shedding. Shedders had shorter PFS compared to non-shedders (p = 0.03). Patients with detectable mEGFR in ctDNA at the first radiological assessment exhibited worse PFS compared to patients with ctDNA clearance (p = 0.05).
Conclusion: Our preliminary data demonstrate that specific clinical characteristics predict mEGFR shedding in ctDNA of NSCLC, suggesting a potential clinical applicability for understanding potential false negative results and appropriate reporting in clinical practice.
Keywords: Circulating-tumor DNA; NSCLC; Predictive biomarkers; Tumor shedding; mEGFR.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Romano Danesi reports that financial support was provided by the Ministry of University and Research, Rome, Italy. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Rosell R., et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009;361(10):958–967. - PubMed
-
- Suda K., Mitsudomi T. Role of EGFR mutations in lung cancers: prognosis and tumor chemosensitivity. Arch. Toxicol. 2015;89(8):1227–1240. - PubMed
-
- Planchard D., et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29(4):iv192–iv237. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

